

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM68391 (2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hydroxymethyl)oxolane-3,4-diol::(2R,3S,4S,5R)-2-(6-amino-2-fluoro-purin-9-yl)-5-methylol-tetrahydrofuran-3,4-diol::(2R,3S,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol::(2R,3S,4S,5R)-2-(6-azanyl-2-fluoranyl-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol::FLUDARABINE::FLUDARABINE PHOSPHATE::MLS000028687::SMR000058874::cid_657237::fludara
SMILES: Nc1nc(F)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O
InChI Key: InChIKey=HBUBKKRHXORPQB-FJFJXFQQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM68391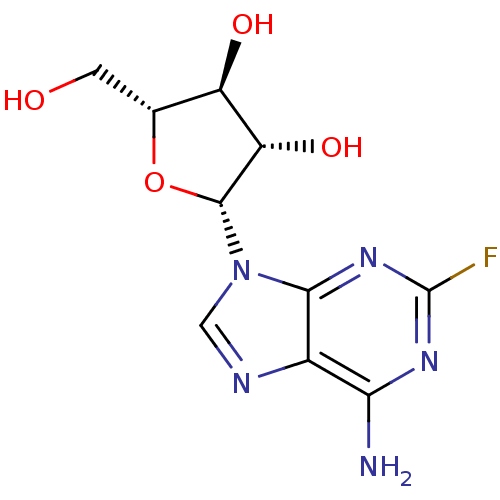 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor in rat brain membranes by measuring displacement of specific [3H]PIA as radioligand. | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity to adenosine A2A receptor in rat striatal membranes by measuring displacement of specific [3H]-CGS- 21680 as radioligand | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a and A3 (Rattus norvegicus) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity determined by displacement of specific binding of [125I]N-(4-amino-3-iodophenethyl)-adenosine in membranes of CHO cells stably trans... | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive type of inhibition of human 5'-nucleotidase using IMP as substrate assessed as inhibitor constant for free enzyme by Lineweaver-Burk plot... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Non-competitive type of inhibition of human 5'-nucleotidase using IMP as substrate assessed as inhibitor constant for enzyme substrate complex by Lin... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| corticotropin releasing factor-binding protein (Homo sapiens (Human)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >5.30E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2VX0F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: GSK3beta, dose response, kinase, inhibition, HTS Assay Overview: The glycogen synthase kinase-3 beta (GSK-3b) is a known master regulator f... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2TX3CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||