

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM728 (3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-3-oxo-4-(1-oxo-1,2,3,4-tetrahydroisoquinolin-4-yl)-2,3-dihydro-1H-pyrrol-2-yl]-3-hydroxy-1-phenylbutan-2-yl]carbamate::Pyrrolinone deriv. 9::Pyrrolinone inhibitor 9::Tetrahydroisoquinolyl Inhibitor::monopyrrolinone-based inhibitor (+)-3::monopyrrolinone-based inhibitor 3
SMILES: O[C@@H](C[C@]1(Cc2ccccc2)N=CC(C2CNC(=O)c3ccccc23)C1=O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1
InChI Key: InChIKey=RROHLUJAQRVJPX-GHJDJLJMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM728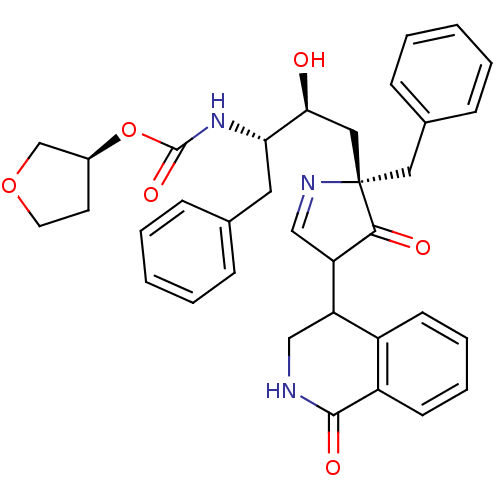 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-3-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM728 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-3-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||