

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM766 Cyclooctylpyranonesulfonamide deriv. 8e::N-{3-[cyclopropyl({4-hydroxy-2-oxo-2H,5H,6H,7H,8H,9H,10H-cycloocta[b]pyran-3-yl})methyl]phenyl}-4-methylbenzene-1-sulfonamide::Sulfonamide-Substituted Cyclooctylpyranone deriv. 35a::U-103017 Analog
SMILES: Cc1ccc(cc1)S(=O)(=O)Nc1cccc(c1)C(C1CC1)c1c(O)c2CCCCCCc2oc1=O
InChI Key: InChIKey=WMWRKGRHVTXXNA-UHFFFAOYSA-N
Data: 3 KI
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM766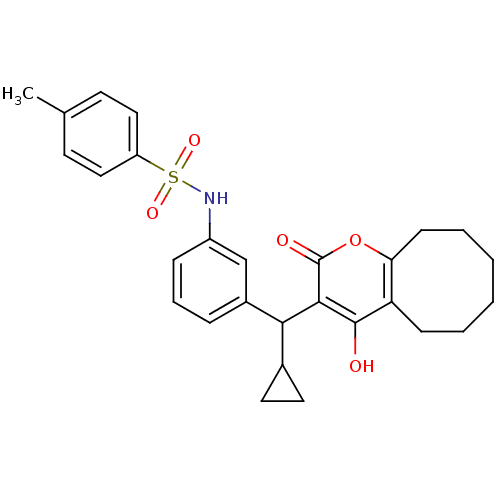 (Cyclooctylpyranonesulfonamide deriv. 8e | N-{3-[cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -11.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 38: 4968-71 (1995) Article DOI: 10.1021/jm00026a002 BindingDB Entry DOI: 10.7270/Q2ZP449D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM766 (Cyclooctylpyranonesulfonamide deriv. 8e | N-{3-[cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease. | Bioorg Med Chem Lett 7: 399-402 (1997) Article DOI: 10.1016/S0960-894X(97)00031-0 BindingDB Entry DOI: 10.7270/Q21R6QH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM766 (Cyclooctylpyranonesulfonamide deriv. 8e | N-{3-[cy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30 | -11.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 40: 1149-64 (1997) Article DOI: 10.1021/jm960441m BindingDB Entry DOI: 10.7270/Q2736P28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||