

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM79180 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylic acid O3-ethyl ester O5-methyl ester;benzenesulfonic acid::2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylic acid O3-ethyl ester O5-methyl ester;besylic acid::AMLODIPINE::MLS001331726::SMR000814710::benzenesulfonic acid;3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate::benzenesulfonic acid;O3-ethyl O5-methyl 2-(2-azanylethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate::cid_60496
SMILES: CCOC(=O)C1=C(COCCN)N=C(C)C(C1c1ccccc1Cl)C(=O)OC
InChI Key: InChIKey=YMDXSGBNCBQYGC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium channel (RAT) | BDBM79180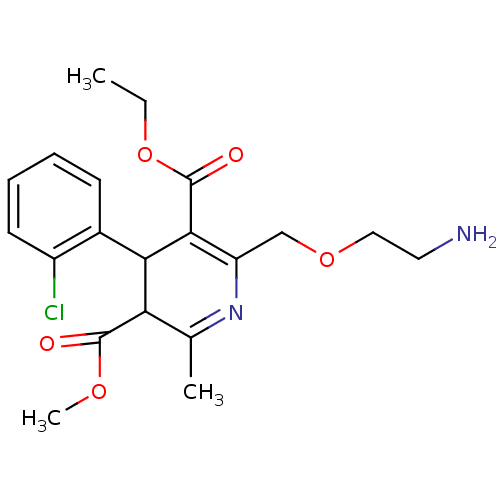 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against rat L-type calcium channel | Bioorg Med Chem Lett 9: 2843-8 (1999) BindingDB Entry DOI: 10.7270/Q2NK3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1a adrenergic receptor | Bioorg Med Chem Lett 9: 2843-8 (1999) BindingDB Entry DOI: 10.7270/Q2NK3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1d adrenergic receptor | Bioorg Med Chem Lett 9: 2843-8 (1999) BindingDB Entry DOI: 10.7270/Q2NK3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic alpha1B (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1b adrenergic receptor | Bioorg Med Chem Lett 9: 2843-8 (1999) BindingDB Entry DOI: 10.7270/Q2NK3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2X34VX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Twik-RElated Potassium (K+) channel 1 (TREK1) (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of TREK-1 (unknown origin) | J Med Chem 56: 593-624 (2013) Article DOI: 10.1021/jm3011433 BindingDB Entry DOI: 10.7270/Q23B61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM79180 (2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Daunorubicin transepithelial transport (basal to apical) (Daunorubicin: 0.035 uM) in MDR1-expressing LLC-PK1 cells | Pharm Res 17: 1189-97 (2000) BindingDB Entry DOI: 10.7270/Q2DR2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||