

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: N=C1NC(=O)\C(S1)=C/c1c[nH]c(=O)c(c1)-c1ccc(nc1)N1CCNCC1
InChI Key: InChIKey=BRYJBRLRAMEQPS-RIYZIHGNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homeodomain-interacting protein kinase 2 (Homo sapiens (Human)) | BDBM81551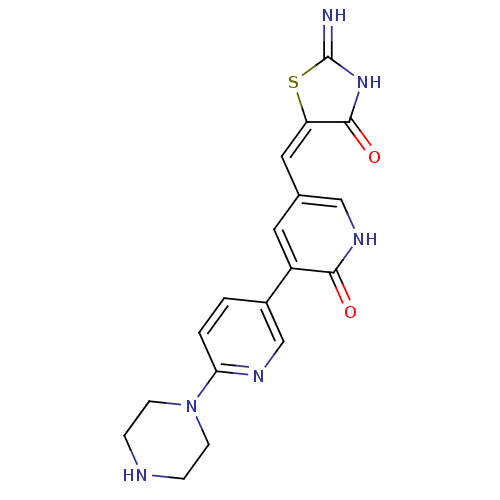 (Furan thiazolidinediones, A64) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | 9.5 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||