

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM833 Benzocycloalkyl Amines deriv. 3::Benzocycloalkyl Amines deriv. 4::CHEMBL439725::tert-butyl N-[(2S,3S,5R)-5-benzyl-5-(2,3-dihydro-1H-inden-2-ylcarbamoyl)-3-hydroxy-1-phenylpentan-2-yl]carbamate
SMILES: CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)NC1Cc2ccccc2C1
InChI Key: InChIKey=YTVUVKHREDAUGZ-GGCFSWKVSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM833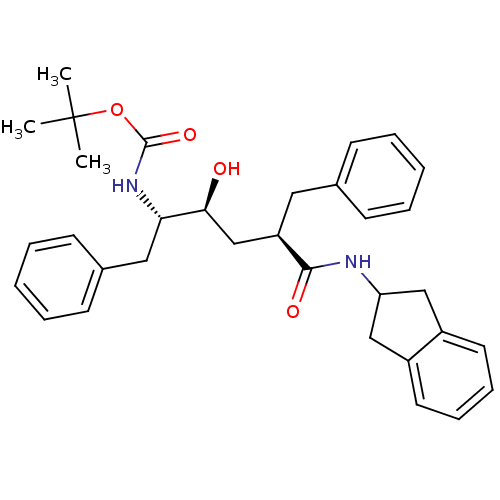 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1228-30 (1991) Article DOI: 10.1021/jm00107a051 BindingDB Entry DOI: 10.7270/Q2XS5SKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM833 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1228-30 (1991) Article DOI: 10.1021/jm00107a051 BindingDB Entry DOI: 10.7270/Q2XS5SKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM833 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibition of human immunodeficiency virus type 1 (HIV-1) protease enzyme. | J Med Chem 43: 4446-51 (2000) BindingDB Entry DOI: 10.7270/Q2S75HMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM833 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease. | J Med Chem 43: 3020-32 (2000) BindingDB Entry DOI: 10.7270/Q2HM59P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM833 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£ Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease. | J Med Chem 41: 836-52 (1998) Article DOI: 10.1021/jm970535b BindingDB Entry DOI: 10.7270/Q21R6RTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM833 (Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||