 Found 8 hits for monomerid = 8344
Found 8 hits for monomerid = 8344 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM8344
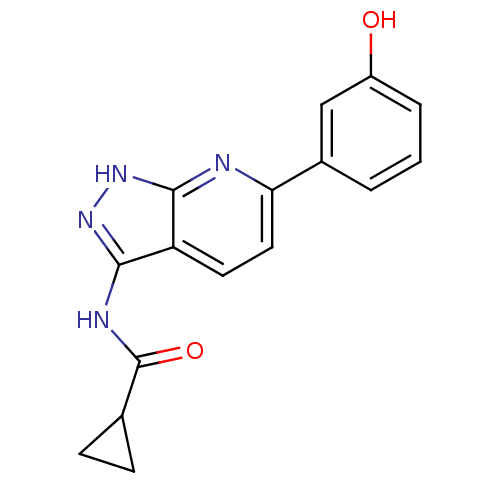
(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 3055-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00645-0
BindingDB Entry DOI: 10.7270/Q26T0JVK |
More data for this
Ligand-Target Pair | |
Cyclin-Dependent Kinase 2 (CDK2)
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 13: 3059-62 (2003)
Article DOI: 10.1016/s0960-894x(03)00646-2
BindingDB Entry DOI: 10.7270/Q2348HKS |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human SKMLCK |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta |
J Med Chem 51: 2062-77 (2008)
Article DOI: 10.1021/jm7009765
BindingDB Entry DOI: 10.7270/Q20Z744C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK1
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human MARK1 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PIM2 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase STK16 (STK16)
(Homo sapiens (Human)) | BDBM8344

(6-aryl-pyrazolo[3,4-b]pyridine analogue 8 | CHEMBL...)Show InChI InChI=1S/C16H14N4O2/c21-11-3-1-2-10(8-11)13-7-6-12-14(17-13)19-20-15(12)18-16(22)9-4-5-9/h1-3,6-9,21H,4-5H2,(H2,17,18,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human MPSK1 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data