

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM85011 AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH2
SMILES: CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O
InChI Key: InChIKey=CEYQBPRBYSKBTL-OSKZNUBXSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSTR2 (RAT) | BDBM85011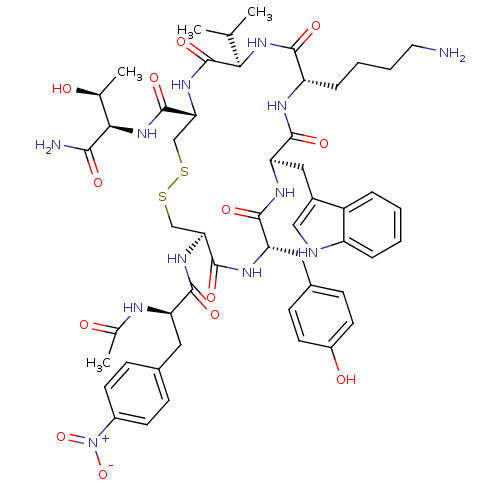 (AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center Curated by PDSP Ki Database | Mol Pharmacol 50: 709-15 (1996) BindingDB Entry DOI: 10.7270/Q2QZ28HK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SSTR5 (RAT) | BDBM85011 (AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center Curated by PDSP Ki Database | Mol Pharmacol 50: 709-15 (1996) BindingDB Entry DOI: 10.7270/Q2QZ28HK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SSTR3 (RAT) | BDBM85011 (AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center Curated by PDSP Ki Database | Mol Pharmacol 50: 709-15 (1996) BindingDB Entry DOI: 10.7270/Q2QZ28HK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||