 Found 19 hits for monomerid = 86282
Found 19 hits for monomerid = 86282 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(RAT) | BDBM86282
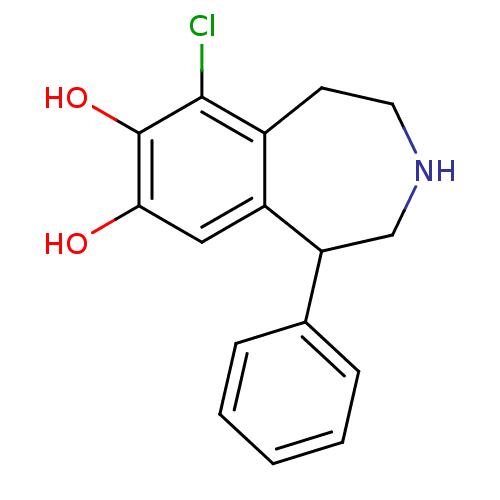
(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 474: 137-40 (2003)
Article DOI: 10.1016/s0014-2999(03)02008-9
BindingDB Entry DOI: 10.7270/Q2DN43MQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at dopamine receptor D1 |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... |
ACS Med Chem Lett 10: 792-799 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00050
BindingDB Entry DOI: 10.7270/Q26W9FKV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity towards dopamine receptor D2 at high affinity state. |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 509 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 474: 137-40 (2003)
Article DOI: 10.1016/s0014-2999(03)02008-9
BindingDB Entry DOI: 10.7270/Q2DN43MQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 995 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 474: 137-40 (2003)
Article DOI: 10.1016/s0014-2999(03)02008-9
BindingDB Entry DOI: 10.7270/Q2DN43MQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 474: 137-40 (2003)
Article DOI: 10.1016/s0014-2999(03)02008-9
BindingDB Entry DOI: 10.7270/Q2DN43MQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity towards dopamine receptor D2 at low affinity state. |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 474: 137-40 (2003)
Article DOI: 10.1016/s0014-2999(03)02008-9
BindingDB Entry DOI: 10.7270/Q2DN43MQ |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... |
ACS Med Chem Lett 10: 792-799 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00050
BindingDB Entry DOI: 10.7270/Q26W9FKV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... |
ACS Med Chem Lett 10: 792-799 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00050
BindingDB Entry DOI: 10.7270/Q26W9FKV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human D1 receptor expressed in CHOK1 cells assessed as induction of beta arrestin2 recruitment measured after 30 mins... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galanin receptor type 3
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q270802Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... |
ACS Med Chem Lett 10: 792-799 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00050
BindingDB Entry DOI: 10.7270/Q26W9FKV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q24748HT |
More data for this
Ligand-Target Pair | |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q280517J |
More data for this
Ligand-Target Pair | |
Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2CN72J7 |
More data for this
Ligand-Target Pair | |
Large T antigen
(Simian virus 40) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a |
Southern Research Specialized Biocontainment Screening Center
Curated by PubChem BioAssay
| Assay Description
Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Librarie... |
PubChem Bioassay (2009)
BindingDB Entry DOI: 10.7270/Q2ZK5F47 |
More data for this
Ligand-Target Pair | |
Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM86282

(6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2QZ28KG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data