

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM87059 CHEMBL247767::Chymostatin
SMILES: CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O
InChI Key: InChIKey=MRXDGVXSWIXTQL-HYHFHBMOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin (Homo sapiens (Human)) | BDBM87059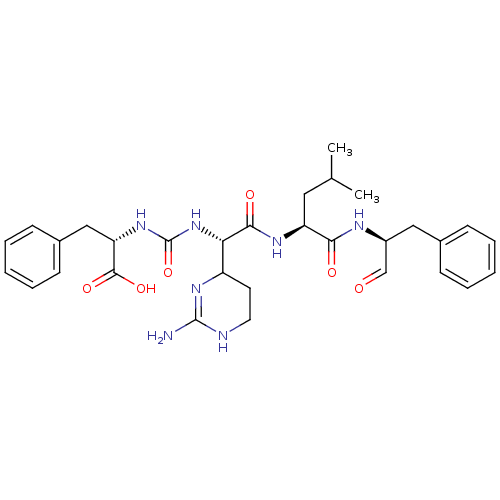 (CHEMBL247767 | Chymostatin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description inhibitory activity against alpha chymotrypsin from bovine pancreas. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description inhibitory activity was evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.24E+3 | -6.93 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi | Assay Description Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. | J Enzyme Inhib Med Chem 23: 400-5 (2008) Article DOI: 10.1080/14756360701584653 BindingDB Entry DOI: 10.7270/Q2JS9P23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||