

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM88776 2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl-3-keto-7-methyl-5H-thiazolo[3,2-a]pyrimidin-2-ylidene]methyl]phenoxy]acetic acid::2-[4-[(Z)-[5-(4-chlorophenyl)-7-methyl-3-oxidanylidene-6-propan-2-yloxycarbonyl-5H-[1,3]thiazolo[3,2-a]pyrimidin-2-ylidene]methyl]phenoxy]ethanoic acid::2-[4-[(Z)-[5-(4-chlorophenyl)-7-methyl-3-oxo-6-[oxo(propan-2-yloxy)methyl]-5H-thiazolo[3,2-a]pyrimidin-2-ylidene]methyl]phenoxy]acetic acid::2-[4-[(Z)-[5-(4-chlorophenyl)-7-methyl-3-oxo-6-propan-2-yloxycarbonyl-5H-[1,3]thiazolo[3,2-a]pyrimidin-2-ylidene]methyl]phenoxy]acetic acid::MLS001178474::SMR000477248::cid_5777336::{4-[(5-(4-chlorophenyl)-6-(isopropoxycarbonyl)-7-methyl-3-oxo-5H-[1,3]thiazolo[3,2-a]pyrimidin-2(3H)-ylidene)methyl]phenoxy}acetic acid
SMILES: CC(C)OC(=O)C1=C(C)N=c2s\c(=C/c3ccc(OCC(O)=O)cc3)c(=O)n2C1c1ccc(Cl)cc1
InChI Key: InChIKey=IOSUGCLQHJKTCK-NDENLUEZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematopoietic protein-tyrosine phosphatase (HEPTP) (Homo sapiens (Human)) | BDBM88776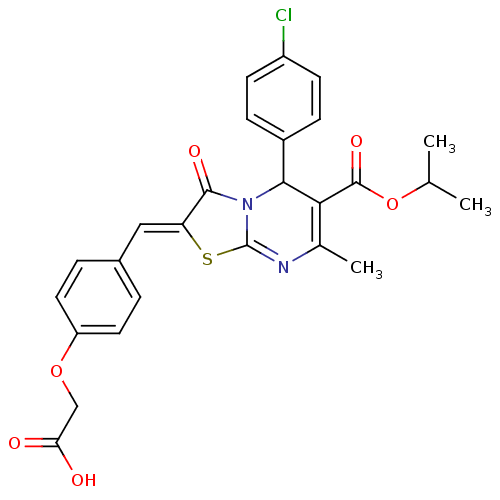 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of recombinant HePTP expressed in Escherichia coli by Michaelis-Menten kinetic analysis | ACS Med Chem Lett 2: 113-118 (2011) Article DOI: 10.1021/ml100103p BindingDB Entry DOI: 10.7270/Q2319WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2959G44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2T43RPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| tyrosine-protein phosphatase non-receptor type 5 isoform a (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2KH0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein 1 (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of KwaZulu-Natal (UKZN) Curated by ChEMBL | Assay Description Inhibition EG5 (unknown origin) | Bioorg Med Chem Lett 28: 2930-2938 (2018) Article DOI: 10.1016/j.bmcl.2018.07.007 BindingDB Entry DOI: 10.7270/Q2542R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic protein-tyrosine phosphatase (HEPTP) (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HePTP expressed in Escherichia coli | ACS Med Chem Lett 2: 113-118 (2011) Article DOI: 10.1021/ml100103p BindingDB Entry DOI: 10.7270/Q2319WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant VHR expressed in Escherichia coli | ACS Med Chem Lett 2: 113-118 (2011) Article DOI: 10.1021/ml100103p BindingDB Entry DOI: 10.7270/Q2319WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 6 (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant MKP3 expressed in Escherichia coli | ACS Med Chem Lett 2: 113-118 (2011) Article DOI: 10.1021/ml100103p BindingDB Entry DOI: 10.7270/Q2319WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| tyrosine-protein phosphatase non-receptor type 5 isoform a (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2DV1HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic cell protein-tyrosine phosphatase 70Z-PEP (Homo sapiens (Human)) | BDBM88776 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2FQ9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||