

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8890 3-(5-Methoxy-1H-inden-2-yl)pyridine::indene 3
SMILES: COc1ccc2CC(=Cc2c1)c1cccnc1
InChI Key: InChIKey=KPJYOCDWGFDTFR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM8890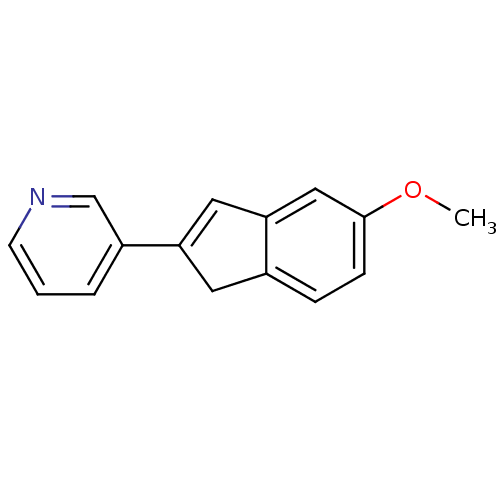 (3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | Pharmacol Rev 49: 2222-31 (2006) Article DOI: 10.1021/jm060055x BindingDB Entry DOI: 10.7270/Q2MW2FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM8890 (3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of aromatase | J Med Chem 51: 2481-91 (2008) Article DOI: 10.1021/jm701314u BindingDB Entry DOI: 10.7270/Q2GX4CF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM8890 (3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | Pharmacol Rev 49: 2222-31 (2006) Article DOI: 10.1021/jm060055x BindingDB Entry DOI: 10.7270/Q2MW2FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8890 (3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.68E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | Pharmacol Rev 49: 2222-31 (2006) Article DOI: 10.1021/jm060055x BindingDB Entry DOI: 10.7270/Q2MW2FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM8890 (3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re... | Pharmacol Rev 49: 2222-31 (2006) Article DOI: 10.1021/jm060055x BindingDB Entry DOI: 10.7270/Q2MW2FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||