

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8909 6-(pyridin-3-yl)naphthalene-2-carbonitrile::6-Pyridin-3-yl-2-naphthonitrile::CHEMBL196796::Pyridine-substituted naphthalene 8::US9271963, 8
SMILES: N#Cc1ccc2cc(ccc2c1)-c1cccnc1
InChI Key: InChIKey=BRNSTMOAGOETDI-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8909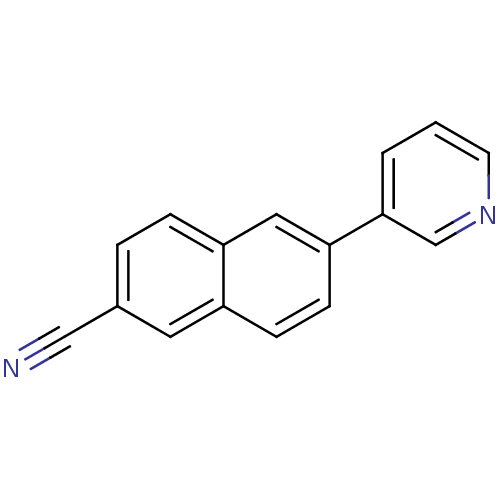 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 691 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 6632-42 (2005) Article DOI: 10.1021/jm0503704 BindingDB Entry DOI: 10.7270/Q2H41PNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 6632-42 (2005) Article DOI: 10.1021/jm0503704 BindingDB Entry DOI: 10.7270/Q2H41PNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 6632-42 (2005) Article DOI: 10.1021/jm0503704 BindingDB Entry DOI: 10.7270/Q2H41PNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 686 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 6632-42 (2005) Article DOI: 10.1021/jm0503704 BindingDB Entry DOI: 10.7270/Q2H41PNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental CYP19 | J Med Chem 51: 8077-87 (2008) Article DOI: 10.1021/jm800888q BindingDB Entry DOI: 10.7270/Q23B6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 37 |
UNIVERSITAT DES SAARLANDES US Patent | Assay Description V79 MZh11B1 and V79 MZh 11B2 cells (8˙10^5 cells per well) were grown to confluency on 24-well cell culture plates with 1.9 cm^2 culture area per... | US Patent US9271963 (2016) BindingDB Entry DOI: 10.7270/Q2445KBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 686 | n/a | n/a | n/a | n/a | 7.4 | 37 |
UNIVERSITAT DES SAARLANDES US Patent | Assay Description A solution of 6.25 nmol of progesterone (in 5 μl of MeOH) was dissolved in 140 μl of phosphate buffer (0.05 M; pH 7.4; 1 mM MgCl2; 0.1 mM EDTA ... | US Patent US9271963 (2016) BindingDB Entry DOI: 10.7270/Q2445KBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 691 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8909 (6-(pyridin-3-yl)naphthalene-2-carbonitrile | 6-Pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 691 | n/a | n/a | n/a | n/a | n/a | 37 |
UNIVERSITAT DES SAARLANDES US Patent | Assay Description V79 MZh11B1 and V79 MZh 11B2 cells (8˙10^5 cells per well) were grown to confluency on 24-well cell culture plates with 1.9 cm^2 culture area per... | US Patent US9271963 (2016) BindingDB Entry DOI: 10.7270/Q2445KBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||