

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9018 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide::N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide::Tacrine-Melatonin Hybrid 11a
SMILES: Oc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4ccccc34)c2c1
InChI Key: InChIKey=DIHZVMMPFILOAT-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9018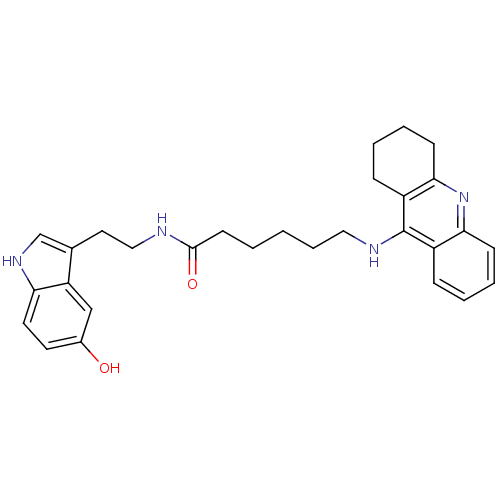 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||