

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9022 CHEMBL225567::Indole-Tacrine Heterodimer 5::N-[5-(6-Chloro-1,2,3,4-tetrahydroacridin-9-ylamino)-hexyl]-3-(1H-indol-3-yl)propionamide::N-{6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]hexyl}-3-(1H-indol-3-yl)propanamide
SMILES: Clc1ccc2c(NCCCCCCNC(=O)CCc3c[nH]c4ccccc34)c3CCCCc3nc2c1
InChI Key: InChIKey=CPLGYLJHIKGTTM-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9022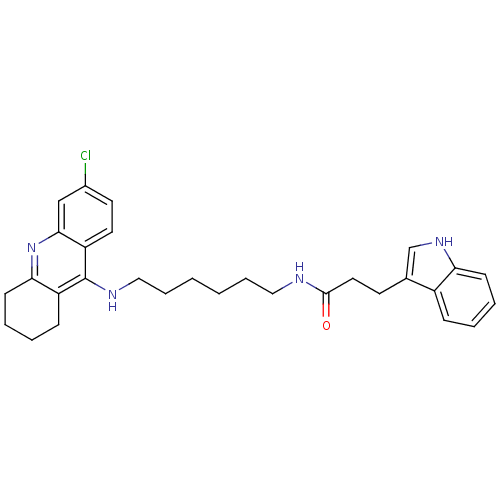 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9022 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human AchE-induced amyloid beta aggregation | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9022 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human AchE | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM9022 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||