

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9027 3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahydroacridin-9-ylamino)propyl]amino}propyl)propanamide::Indole-Tacrine Heterodimer 10::N-(3-{[3-(1,2,3,4-Tetrahydroacridin-9-ylamino)propyl]-methylamino}propyl)-3-(1H-indol-3-yl)propionamide
SMILES: CN(CCCNC(=O)CCc1c[nH]c2ccccc12)CCCNc1c2CCCCc2nc2ccccc12
InChI Key: InChIKey=YIFIESCJSCAHBP-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9027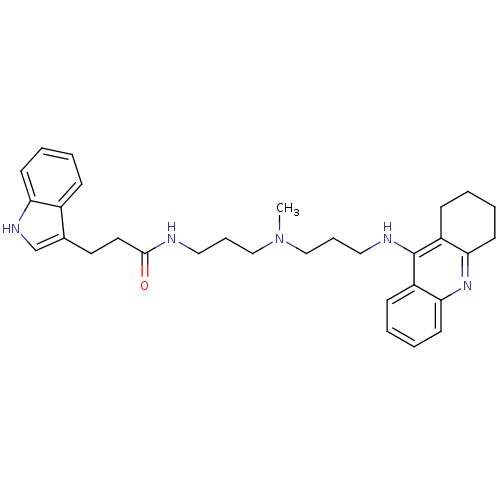 (3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9027 (3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE | Eur J Med Chem 45: 1167-72 (2010) Article DOI: 10.1016/j.ejmech.2009.12.038 BindingDB Entry DOI: 10.7270/Q25H7GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM9027 (3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||