

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM914 Aminodiol deriv. 9a::BMS-186318 analog 1::[(S,R)-3-[[(R,S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester::tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-phenylbutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
SMILES: CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C
InChI Key: InChIKey=KKRYDPVDJYCEER-QEGGNFSNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM914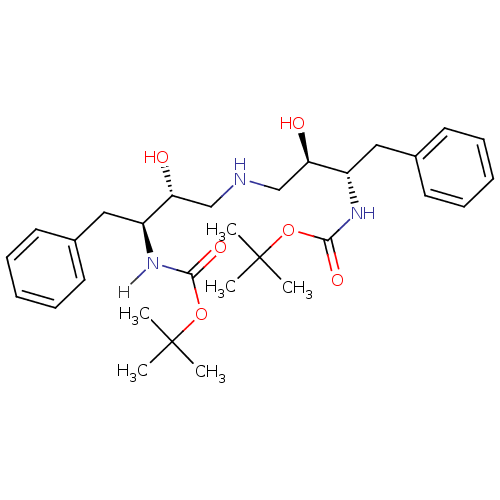 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 100 | -9.93 | 125 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 37: 1758-68 (1994) Article DOI: 10.1021/jm00038a005 BindingDB Entry DOI: 10.7270/Q257196C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM914 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 1729-1734 (1995) Article DOI: 10.1016/0960-894X(95)00293-3 BindingDB Entry DOI: 10.7270/Q2RN37TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM914 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 1729-1734 (1995) Article DOI: 10.1016/0960-894X(95)00293-3 BindingDB Entry DOI: 10.7270/Q2RN37TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM914 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM914 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V | Bioorg Med Chem Lett 5: 459-464 (1995) Article DOI: 10.1016/0960-894X(95)00056-Y BindingDB Entry DOI: 10.7270/Q2RX9C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||