

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9408 (2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-phenylbenzamide::CHEMBL544629::CHEMBL55312::N-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-N-phenyl-benzamide (C4H4O4)::N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-phenylbenzamide Fumarate::Piperidine Derivative 20
SMILES: O=C(N(CCC1CCN(Cc2ccccc2)CC1)c1ccccc1)c1ccccc1
InChI Key: InChIKey=RSMDWTVKHVTHAL-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408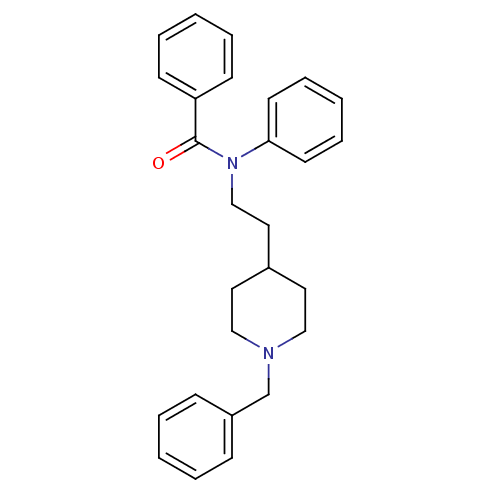 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35.0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibition against Acetylcholinesterase (AChE) | J Med Chem 39: 380-7 (1996) Article DOI: 10.1021/jm950704x BindingDB Entry DOI: 10.7270/Q25D8T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9408 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE | Eur J Med Chem 45: 1167-72 (2010) Article DOI: 10.1016/j.ejmech.2009.12.038 BindingDB Entry DOI: 10.7270/Q25H7GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||