

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 307.2 BDBM50075050 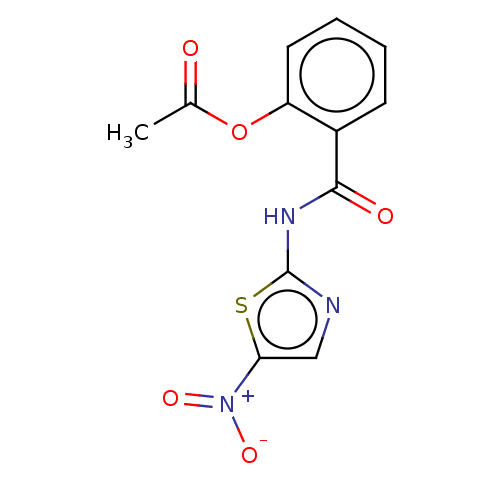 Purchase Purchase | Wt: 314.4 BDBM50239468  Purchase Purchase | Wt: 301.7 BDBM50503432  | Wt: 318.7 BDBM50503437 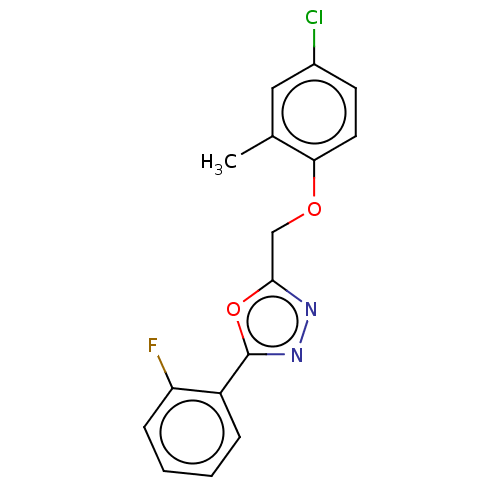 | Wt: 318.7 BDBM50503438 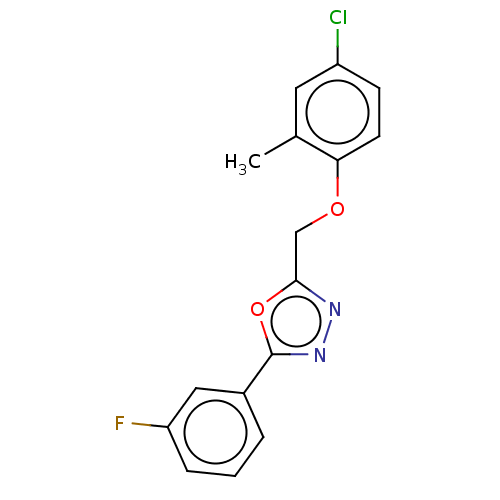 |
| Wt: 290.7 BDBM50503442 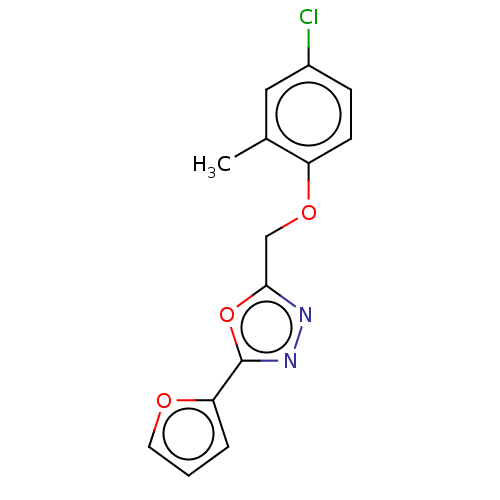 | Wt: 292.7 BDBM50503443 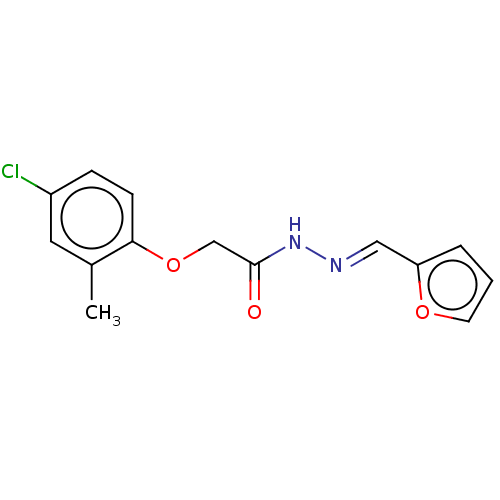 Purchase Purchase | Wt: 303.7 BDBM50503449 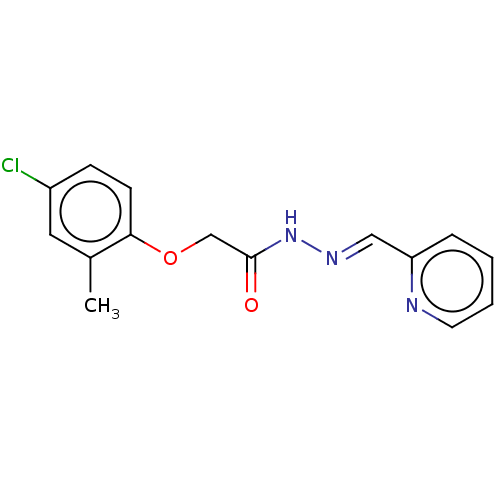 Purchase Purchase | Wt: 313.3 BDBM509668  | Wt: 310.3 BDBM509603  |
| Wt: 317.4 BDBM509606  | Wt: 252.3 BDBM50556197 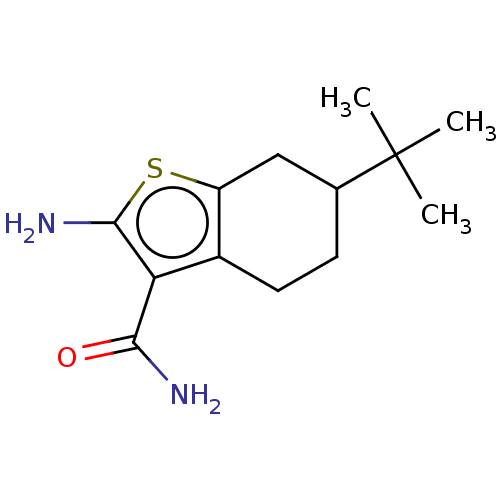 Purchase Purchase | Wt: 294.4 BDBM50556198 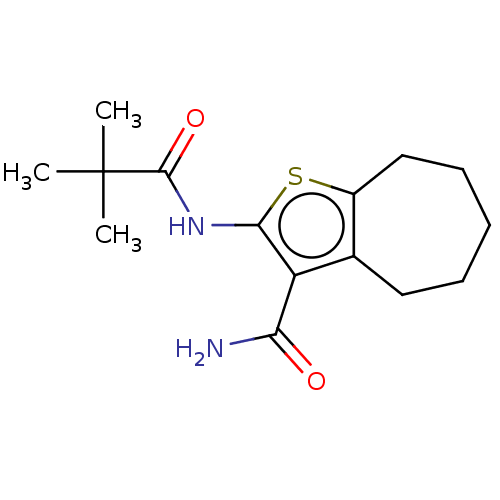 | Wt: 319.7 BDBM542066 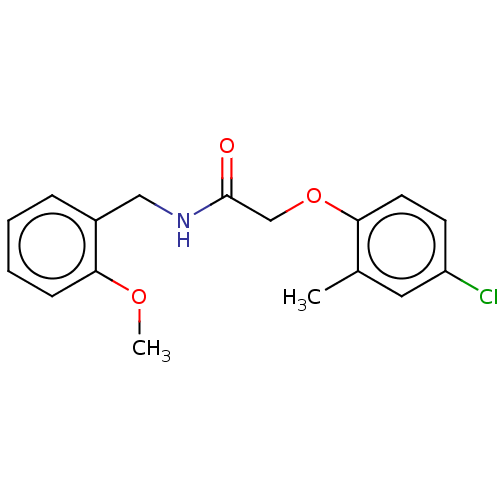 | Wt: 318.7 BDBM50594048  |
| Displayed 1 to 15 (of 392 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate-flavodoxin oxidoreductase (Campylobacter jejuni subsp. jejuni) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Health Systems Curated by ChEMBL | Assay Description Inhibition of PFOR Campylobacter jejuni | Antimicrob Agents Chemother 51: 868-76 (2007) Article DOI: 10.1128/aac.01159-06 BindingDB Entry DOI: 10.7270/Q2JM2DDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114413 BindingDB Entry DOI: 10.7270/Q2GX4GK6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50594048 (CHEMBL5190831) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114413 BindingDB Entry DOI: 10.7270/Q2GX4GK6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Mus musculus) | BDBM50556197 (CHEMBL1505295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GFP-fused mouse ANO1 channel expressed in HEK293A cells assessed as inhibition of ATP-induced channel current amplitude at membrane pot... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112688 BindingDB Entry DOI: 10.7270/Q2N58R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50556197 (CHEMBL1505295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ANO1 channel expressed in HEK293 cells incubated for 10 mins by FLIPR assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112688 BindingDB Entry DOI: 10.7270/Q2N58R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Mus musculus) | BDBM50556198 (CHEMBL4796667) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GFP-fused mouse ANO1 channel expressed in HEK293A cells assessed as inhibition of ATP-induced channel current amplitude at membrane pot... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112688 BindingDB Entry DOI: 10.7270/Q2N58R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50556198 (CHEMBL4796667) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ANO1 channel expressed in HEK293 cells incubated for 10 mins by FLIPR assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112688 BindingDB Entry DOI: 10.7270/Q2N58R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human AR | J Med Chem 58: 2047-67 (2015) Article DOI: 10.1021/jm500907a BindingDB Entry DOI: 10.7270/Q2D79D4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IL-6 induced STAT3 transcriptional activity in human HEK-Blue IL-6 cells assessed as secreted embryonic alkaline phosphatase reporter g... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00544 BindingDB Entry DOI: 10.7270/Q2T72N4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IL-6 induced STAT3 transcriptional activity in human HEK-Blue IL-6 cells assessed as secreted embryonic alkaline phosphatase reporter g... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00544 BindingDB Entry DOI: 10.7270/Q2T72N4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM542066 (US11274074, Example 11 [Chemical Formula 15]) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50239468 (CHEMBL4073870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University Curated by ChEMBL | Assay Description Inhibition of human TMEM16A expressed in FRT cells co-expressing iodide sensitive fluorescent protein YFP-H148Q/I152L/F46L assessed as reduction in A... | J Med Chem 60: 4626-4635 (2017) Article DOI: 10.1021/acs.jmedchem.7b00020 BindingDB Entry DOI: 10.7270/Q2FT8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM542066 (US11274074, Example 11 [Chemical Formula 15]) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503437 (CHEMBL4516441 | US11274074, Example 4 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503437 (CHEMBL4516441 | US11274074, Example 4 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503443 (CHEMBL4579558 | US11274074, Example 8 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503443 (CHEMBL4579558 | US11274074, Example 8 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503449 (CHEMBL4517769 | US11274074, Example 10 [Chemical F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503449 (CHEMBL4517769 | US11274074, Example 10 [Chemical F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503442 (CHEMBL4453660 | US11274074, Example 9 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503442 (CHEMBL4453660 | US11274074, Example 9 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503449 (CHEMBL4517769 | US11274074, Example 10 [Chemical F...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO2 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503449 (CHEMBL4517769 | US11274074, Example 10 [Chemical F...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503438 (CHEMBL4466337 | US11274074, Comparative Example 6 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503432 (CHEMBL4581421 | US11274074, Comparative Example 11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503437 (CHEMBL4516441 | US11274074, Example 4 [Chemical Fo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO2 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503442 (CHEMBL4453660 | US11274074, Example 9 [Chemical Fo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO2 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503437 (CHEMBL4516441 | US11274074, Example 4 [Chemical Fo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503438 (CHEMBL4466337 | US11274074, Comparative Example 6 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503442 (CHEMBL4453660 | US11274074, Example 9 [Chemical Fo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503438 (CHEMBL4466337 | US11274074, Comparative Example 6 ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO2 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503432 (CHEMBL4581421 | US11274074, Comparative Example 11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-2 (Homo sapiens) | BDBM50503438 (CHEMBL4466337 | US11274074, Comparative Example 6 ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The method for inhibiting the expression of ANO1 (TMEM16A) of the present disclosure includes a step of inhibiting the expression of ANO1 (TMEM16A) i... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GT5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 4 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 3 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 2 (Homo sapiens (Human)) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509606 (N-tert-Butyl-4-[[2-[2-thienyl]acetyl]amino]pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound activity was quantified by measuring the increase in current upon compound addition and expressing this as a percentage increase of baseline... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M32ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509603 (US11364246, Example 4.6 | US20200361871A1, Example...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Cell Culture and PreparationFisher rat thyroid (FRT) cells stably expressing human TMEM16A (TMEM16Aabc variant; Dr Luis Galietta, Insituto Giannina, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509606 (N-tert-Butyl-4-[[2-[2-thienyl]acetyl]amino]pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA | Assay Description Cell Culture and PreparationFisher rat thyroid (FRT) cells stably expressing human TMEM16A (TMEM16Aabc variant; Dr Luis Galietta, Insituto Giannina, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509668 (4-[(2-Cyclopentylacetyl)amino]-N-(1,1-dimethylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
TBA | Assay Description Cell Culture and PreparationFisher rat thyroid (FRT) cells stably expressing human TMEM16A (TMEM16Aabc variant; Dr Luis Galietta, Insituto Giannina, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trehalose-binding lipoprotein LpqY (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50075050 (Alinia | Nitazoxanide | PH-5776) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.54E+4 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00104g BindingDB Entry DOI: 10.7270/Q2TQ65JT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509603 (US11364246, Example 4.6 | US20200361871A1, Example...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound activity was quantified by measuring the increase in current upon compound addition and expressing this as a percentage increase of baseline... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M32ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM509668 (4-[(2-Cyclopentylacetyl)amino]-N-(1,1-dimethylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound activity was quantified by measuring the increase in current upon compound addition and expressing this as a percentage increase of baseline... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M32ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||