Found 1165 hits in this display
Found 1165 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50017698
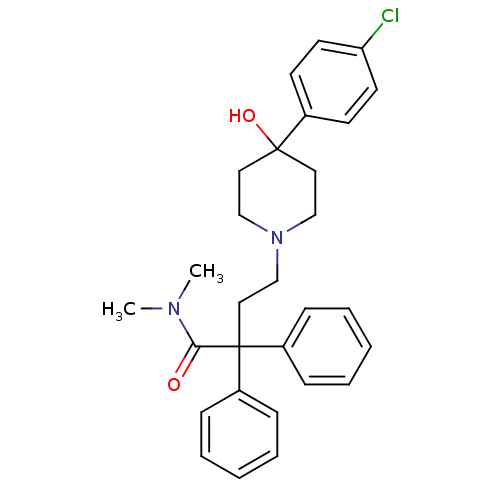
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for Mu opioid receptor of rat brain |
J Med Chem 47: 5009-20 (2004)
Article DOI: 10.1021/jm030548r
BindingDB Entry DOI: 10.7270/Q2H131GF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1403-10 (1994)
BindingDB Entry DOI: 10.7270/Q2154FJQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 120: 365-8 (1995)
Article DOI: 10.1007/bf02311185
BindingDB Entry DOI: 10.7270/Q2VT1QKB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698

(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 28: 519-26 (2003)
Article DOI: 10.1038/sj.npp.1300027
BindingDB Entry DOI: 10.7270/Q2833QKK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas
Curated by PDSP Ki Database
| |
Synapse 38: 438-49 (2000)
Article DOI: 10.1002/1098-2396(20001215)38:4
BindingDB Entry DOI: 10.7270/Q2SN07HX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698

(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 16: 93 (1997)
Article DOI: 10.1016/S0893-133X(96)00187-X
BindingDB Entry DOI: 10.7270/Q2513WR8 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity determined in radioreceptor binding assay by using [3H]ketanserin radioligand against 5-hydroxytryptamine 2 receptor |
J Med Chem 35: 552-8 (1992)
BindingDB Entry DOI: 10.7270/Q2PR7WMW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 1166-78 (2002)
Article DOI: 10.1124/jpet.301.3.1166
BindingDB Entry DOI: 10.7270/Q2V69H4V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Bioorg Med Chem 11: 1451-64 (2003)
Article DOI: 10.1016/S0968-0896(02)00561-8
BindingDB Entry DOI: 10.7270/Q2CV4G90 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Bioorg Med Chem 11: 1451-64 (2003)
Article DOI: 10.1016/S0968-0896(02)00561-8
BindingDB Entry DOI: 10.7270/Q2CV4G90 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
J Med Chem 39: 549-55 (1996)
Article DOI: 10.1021/jm950707+
BindingDB Entry DOI: 10.7270/Q2736PF6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 16: 93 (1997)
Article DOI: 10.1016/S0893-133X(96)00187-X
BindingDB Entry DOI: 10.7270/Q2513WR8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity towards human M1 muscarinic receptor. |
J Med Chem 44: 477-501 (2001)
BindingDB Entry DOI: 10.7270/Q24Q7VQH |
More data for this
Ligand-Target Pair | |
Histone H1.0
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor (unknown origin) |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor |
J Med Chem 48: 1709-12 (2005)
Article DOI: 10.1021/jm049632c
BindingDB Entry DOI: 10.7270/Q2MC8ZHB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 16: 93 (1997)
Article DOI: 10.1016/S0893-133X(96)00187-X
BindingDB Entry DOI: 10.7270/Q2513WR8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 20: 612-27 (1999)
Article DOI: 10.1016/S0893-133X(98)00099-2
BindingDB Entry DOI: 10.7270/Q2ZP44NW |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 1166-78 (2002)
Article DOI: 10.1124/jpet.301.3.1166
BindingDB Entry DOI: 10.7270/Q2V69H4V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methylspiperone from human dopamine D2long receptor expressed in HEK293 cells after 30 mins by scintillation counting |
Bioorg Med Chem 20: 6366-74 (2012)
Article DOI: 10.1016/j.bmc.2012.08.058
BindingDB Entry DOI: 10.7270/Q2G161X3 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Bioorg Med Chem 11: 1451-64 (2003)
Article DOI: 10.1016/S0968-0896(02)00561-8
BindingDB Entry DOI: 10.7270/Q2CV4G90 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by PDSP Ki Database
| |
Life Sci 35: 1885-93 (1984)
Article DOI: 10.1016/0024-3205(84)90540-x
BindingDB Entry DOI: 10.7270/Q2JD4V9M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranes |
J Med Chem 30: 1631-5 (1987)
BindingDB Entry DOI: 10.7270/Q2FB51ZZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 120: 365-8 (1995)
Article DOI: 10.1007/bf02311185
BindingDB Entry DOI: 10.7270/Q2VT1QKB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1403-10 (1994)
BindingDB Entry DOI: 10.7270/Q2154FJQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 28: 519-26 (2003)
Article DOI: 10.1038/sj.npp.1300027
BindingDB Entry DOI: 10.7270/Q2833QKK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Eur J Pharmacol 291: 59-66 (1995)
Article DOI: 10.1016/0922-4106(95)90125-6
BindingDB Entry DOI: 10.7270/Q2TT4PHV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Ann N Y Acad Sci 537: 435-42 (1988)
Article DOI: 10.1111/j.1749-6632.1988.tb42125.x
BindingDB Entry DOI: 10.7270/Q2X065JH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke
Curated by PDSP Ki Database
| |
Mol Pharmacol 37: 1-6 (1990)
BindingDB Entry DOI: 10.7270/Q2Z60MJ0 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha1 receptor in rat cerebral cortex |
J Med Chem 50: 5103-8 (2007)
Article DOI: 10.1021/jm070516u
BindingDB Entry DOI: 10.7270/Q20G3NW3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells |
J Med Chem 50: 5103-8 (2007)
Article DOI: 10.1021/jm070516u
BindingDB Entry DOI: 10.7270/Q20G3NW3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity determined in radioreceptor binding assay by using [3H]ketanserin radioligand against 5-hydroxytryptamine 2 receptor |
J Med Chem 35: 552-8 (1992)
BindingDB Entry DOI: 10.7270/Q2PR7WMW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from recombinant human D2 receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting ... |
Eur J Med Chem 166: 144-158 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.031
BindingDB Entry DOI: 10.7270/Q2M61PPW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2 receptor expressed in HEK cells incubated for 1 hr by Cheng-Prusoff analysis based microbeta scintillat... |
Eur J Med Chem 180: 383-397 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.027
BindingDB Entry DOI: 10.7270/Q23B63H7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
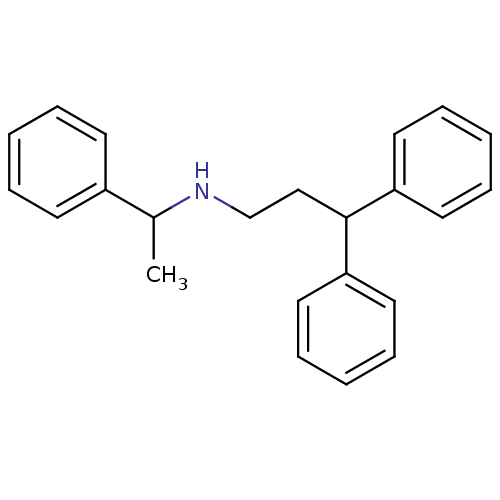 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase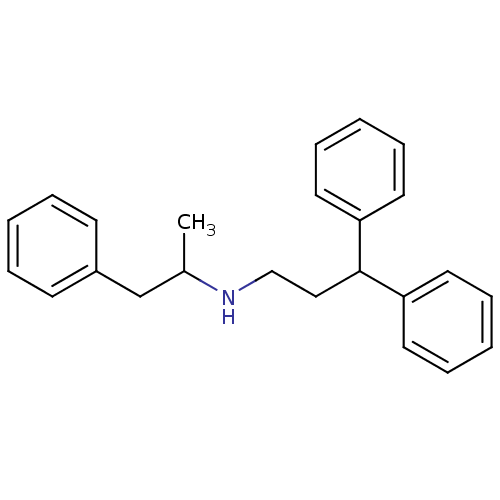 Purchase
Purchase Purchase
Purchase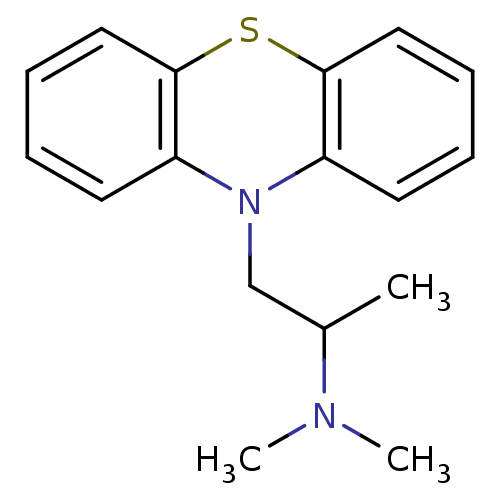 Purchase
Purchase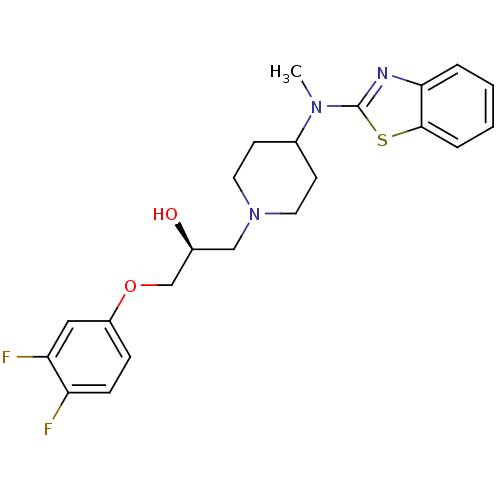 Purchase
Purchase Purchase
Purchase