

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 366.4 BDBM86733  Purchase Purchase | Wt: 382.9 BDBM50060721  | Wt: 368.9 BDBM50060727 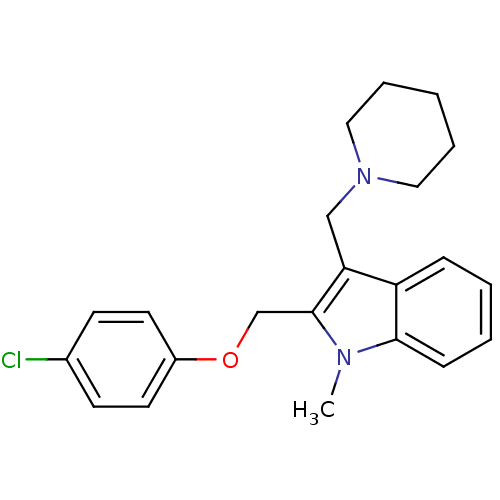 | Wt: 373.4 BDBM50145239  | Wt: 375.3 BDBM50249758 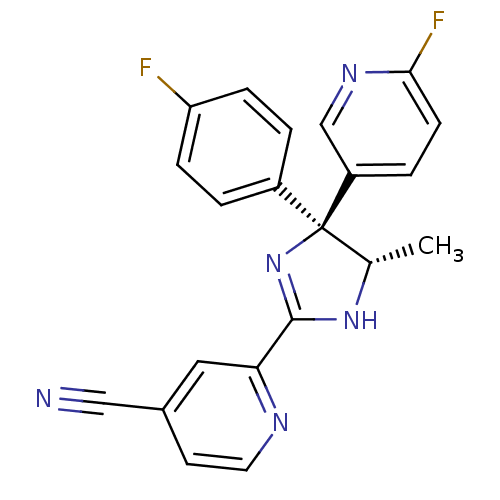 |
| Wt: 388.2 BDBM50264726 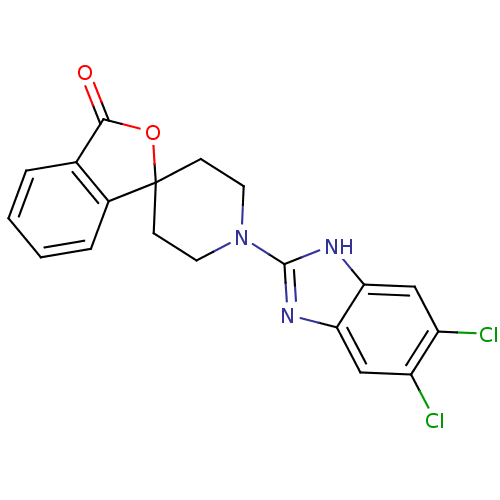 | Wt: 353.8 BDBM50265209  | Wt: 387.3 BDBM50265265 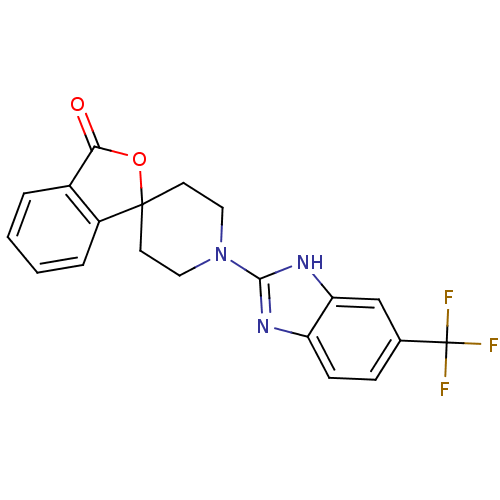 | Wt: 395.4 BDBM50265267  | Wt: 377.3 BDBM50265298 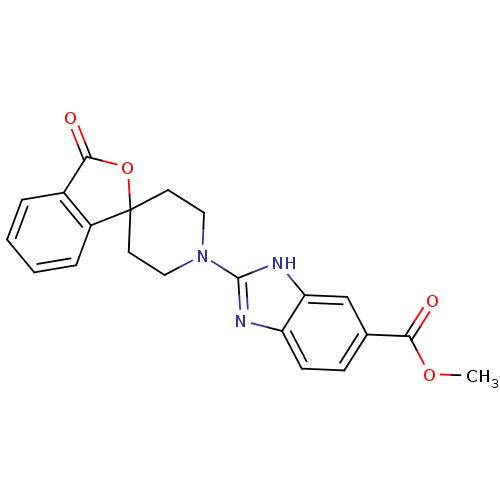 |
| Wt: 354.4 BDBM50312007 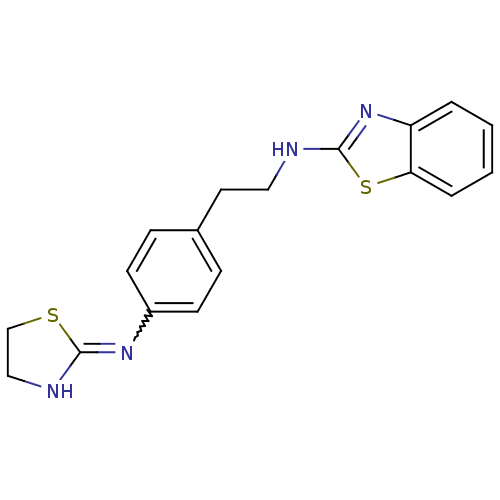 | Wt: 393.4 BDBM50373626  | Wt: 248.3 BDBM50240829 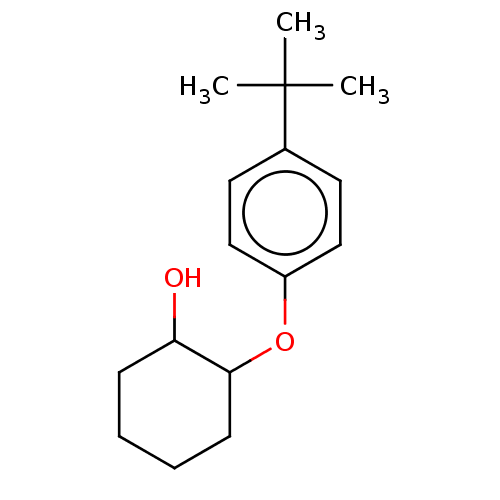 Purchase Purchase | Wt: 397.4 BDBM50563420 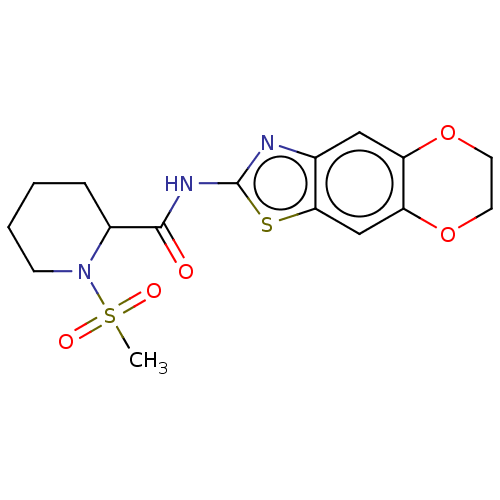 | Wt: 397.4 BDBM50563421 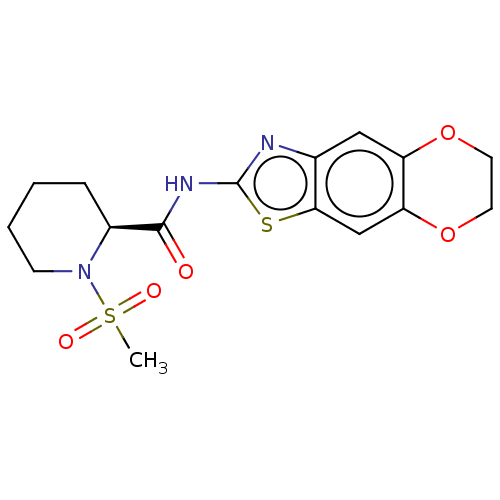 |
| Displayed 1 to 15 (of 304 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50240829 (CHEMBL3559801) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University Curated by ChEMBL | Assay Description Effect on pancreatic polypeptide-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK... | J Med Chem 60: 7605-7612 (2017) Article DOI: 10.1021/acs.jmedchem.7b00976 BindingDB Entry DOI: 10.7270/Q22B915R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPYY5 receptor | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50373626 (CHEMBL257940) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125]PYY from human chimeric NPY Y5 receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 18: 1146-50 (2008) Article DOI: 10.1016/j.bmcl.2007.11.132 BindingDB Entry DOI: 10.7270/Q27D2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060721 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-(2-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50240829 (CHEMBL3559801) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University Curated by ChEMBL | Assay Description Effect on peptide YY-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK293 cell mem... | J Med Chem 60: 7605-7612 (2017) Article DOI: 10.1021/acs.jmedchem.7b00976 BindingDB Entry DOI: 10.7270/Q22B915R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50240829 (CHEMBL3559801) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University Curated by ChEMBL | Assay Description Effect on neuropeptide Y-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK293 cell... | J Med Chem 60: 7605-7612 (2017) Article DOI: 10.1021/acs.jmedchem.7b00976 BindingDB Entry DOI: 10.7270/Q22B915R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in thymidine kinase deficient human LM cells | J Med Chem 51: 4765-70 (2008) Article DOI: 10.1021/jm8003587 BindingDB Entry DOI: 10.7270/Q2J96664 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50312007 (CHEMBL1081469 | N-(4-(4,5-dihydrothiazol-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]pig PPY from human NPY1 receptor expressed in CHOK1 cells by scintillation counting | Bioorg Med Chem Lett 19: 6801-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.048 BindingDB Entry DOI: 10.7270/Q2XP753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060727 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060727 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50060721 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-(2-piperidin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 5 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50373626 (CHEMBL257940) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y4 receptor | Bioorg Med Chem Lett 18: 1146-50 (2008) Article DOI: 10.1016/j.bmcl.2007.11.132 BindingDB Entry DOI: 10.7270/Q27D2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50373626 (CHEMBL257940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y2 receptor | Bioorg Med Chem Lett 18: 1146-50 (2008) Article DOI: 10.1016/j.bmcl.2007.11.132 BindingDB Entry DOI: 10.7270/Q27D2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50373626 (CHEMBL257940) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y1 receptor | Bioorg Med Chem Lett 18: 1146-50 (2008) Article DOI: 10.1016/j.bmcl.2007.11.132 BindingDB Entry DOI: 10.7270/Q27D2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50060721 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-(2-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 4 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50060727 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 5 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50060727 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 4 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50060727 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 2 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50312007 (CHEMBL1081469 | N-(4-(4,5-dihydrothiazol-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]pig PPY from human NPY2 receptor expressed in CHOK1 cells by scintillation counting | Bioorg Med Chem Lett 19: 6801-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.048 BindingDB Entry DOI: 10.7270/Q2XP753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50060721 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-(2-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity for human Neuropeptide Y receptor type 2 | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50312007 (CHEMBL1081469 | N-(4-(4,5-dihydrothiazol-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]pig PPY from human NPY5 receptor expressed in CHOK1 cells by scintillation counting | Bioorg Med Chem Lett 19: 6801-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.048 BindingDB Entry DOI: 10.7270/Q2XP753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50312007 (CHEMBL1081469 | N-(4-(4,5-dihydrothiazol-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]human PP from human NPY4 receptor expressed in CHOK1 cells by scintillation counting | Bioorg Med Chem Lett 19: 6801-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.048 BindingDB Entry DOI: 10.7270/Q2XP753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265209 (1'-(5-chloro-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264726 (1'-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-3H-spir...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265298 (CHEMBL496327 | methyl 2-(3-oxo-3H-spiro[isobenzofu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264726 (1'-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-3H-spir...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265267 (1'-(5-phenyl-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant neuropeptide Y5 receptor expressed in CHO-K1 cells coexpressing Gqi5 assessed as inhibition of NPY-induced i... | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265267 (1'-(5-phenyl-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265298 (CHEMBL496327 | methyl 2-(3-oxo-3H-spiro[isobenzofu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265265 (1'-(5-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265209 (1'-(5-chloro-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant neuropeptide Y5 receptor expressed in CHO cells coexpressing Gqi5 assessed as inhibition of neuropeptide Y-i... | J Med Chem 51: 4765-70 (2008) Article DOI: 10.1021/jm8003587 BindingDB Entry DOI: 10.7270/Q2J96664 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145239 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145239 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor measured as Ca+ response | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50563421 (CHEMBL4745232) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at eFYP-tagged human NPY4R expressed in COS7 cells co-expressing Gqi5-alpha assessed as inhibition of PP-induced Ca2+ ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50563421 (CHEMBL4745232) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at eFYP-tagged human NPY4R expressed in COS7 cells co-expressing Gqi5-alpha assessed as inhibition of PP-induced Ca2+ ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM50563421 (CHEMBL4745232) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at NPY5R in mouse descending colon mucosa assessed as reduction in rPP-induced ion transport by electrophysiology | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM50563421 (CHEMBL4745232) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at NPY5R in mouse descending colon mucosa assessed as reduction in rPP-induced ion transport by electrophysiology | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50563420 (CHEMBL4780125) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at eFYP-tagged human NPY4R expressed in COS7 cells co-expressing Gqi5-alpha assessed as inhibition of PP-induced Ca2+ ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50563420 (CHEMBL4780125) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric antagonist activity at eFYP-tagged human NPY4R expressed in COS7 cells co-expressing Gqi5-alpha assessed as inhibition of PP-induced Ca2+ ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02000 BindingDB Entry DOI: 10.7270/Q24F1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265265 (1'-(5-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50265209 (1'-(5-chloro-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to NPY Y2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50265265 (1'-(5-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to NPY Y2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50265267 (1'-(5-phenyl-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to NPY Y2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |