 Found 177 hits in this display
Found 177 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50366480
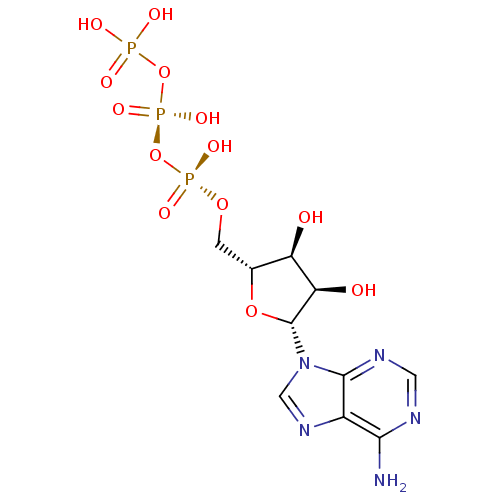
(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of ATP, non competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase type 2-alpha
(Homo sapiens (Human)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity (Ki) against human phosphatidylinositol 4-kinase |
J Med Chem 33: 2073-80 (1990)
BindingDB Entry DOI: 10.7270/Q25T3NQV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycerol kinase
(Homo sapiens (Human)) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against glycerol kinase (ATP competitive inhibition) |
Bioorg Med Chem Lett 7: 2613-2616 (1997)
Article DOI: 10.1016/S0960-894X(97)10051-8
BindingDB Entry DOI: 10.7270/Q2GF0V0S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-2
(Rattus norvegicus) | BDBM50118221
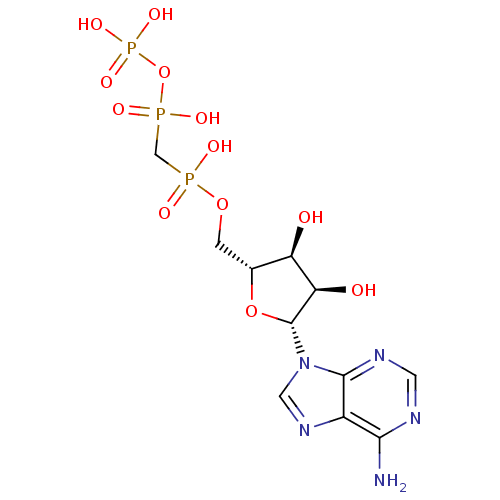
(9H-purine derivative | CHEMBL132722 | DIPHOSPHOMET...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H18N5O12P3/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(27-11)1-26-29(19,20)4-30(21,22)28-31(23,24)25/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,25)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat kidney Methionine adenosyltransferase II |
J Med Chem 29: 318-22 (1986)
BindingDB Entry DOI: 10.7270/Q2DF6RSH |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50118221

(9H-purine derivative | CHEMBL132722 | DIPHOSPHOMET...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H18N5O12P3/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(27-11)1-26-29(19,20)4-30(21,22)28-31(23,24)25/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,25)/t5-,7-,8-,11-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat Methionine adenosyltransferase was reported |
J Med Chem 29: 318-22 (1986)
BindingDB Entry DOI: 10.7270/Q2DF6RSH |
More data for this
Ligand-Target Pair | |
Adenylate kinase isoenzyme 1
(Rattus norvegicus) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat Adenylate kinase M isoenzyme in the presence of ATP non competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
2-dehydropantoate 2-reductase
(Escherichia coli (strain K12)) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli KPR |
J Med Chem 49: 4992-5000 (2006)
Article DOI: 10.1021/jm060490r
BindingDB Entry DOI: 10.7270/Q28S4QQN |
More data for this
Ligand-Target Pair | |
2-dehydropantoate 2-reductase
(Escherichia coli (strain K12)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli KPR |
J Med Chem 49: 4992-5000 (2006)
Article DOI: 10.1021/jm060490r
BindingDB Entry DOI: 10.7270/Q28S4QQN |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50257636
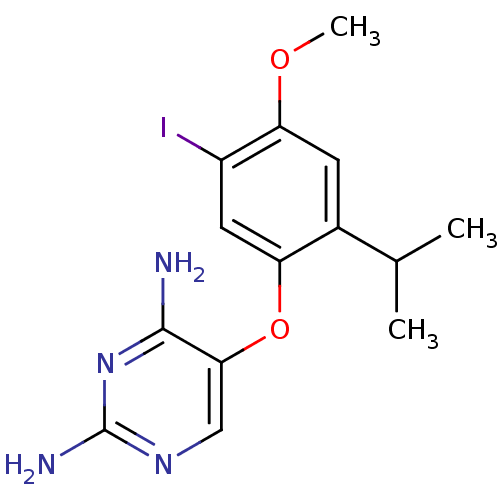
(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of 5HT3 receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of 5HT6 receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of 5HT2A receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50257829
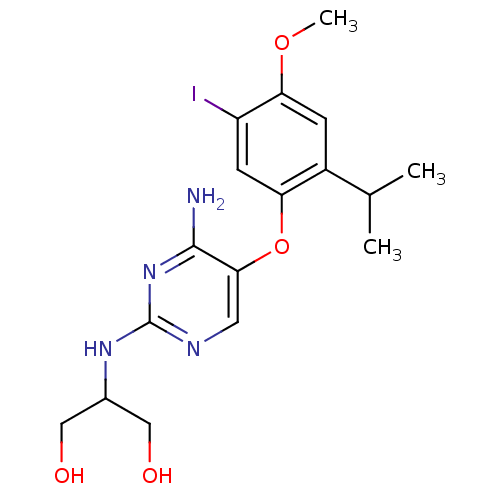
(2-(4-amino-5-(5-iodo-2-isopropyl-4-methoxyphenoxy)...)Show InChI InChI=1S/C17H23IN4O4/c1-9(2)11-4-14(25-3)12(18)5-13(11)26-15-6-20-17(22-16(15)19)21-10(7-23)8-24/h4-6,9-10,23-24H,7-8H2,1-3H3,(H3,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50257829

(2-(4-amino-5-(5-iodo-2-isopropyl-4-methoxyphenoxy)...)Show InChI InChI=1S/C17H23IN4O4/c1-9(2)11-4-14(25-3)12(18)5-13(11)26-15-6-20-17(22-16(15)19)21-10(7-23)8-24/h4-6,9-10,23-24H,7-8H2,1-3H3,(H3,19,20,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X2/3 receptor expressed in 1321n1c cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50257829

(2-(4-amino-5-(5-iodo-2-isopropyl-4-methoxyphenoxy)...)Show InChI InChI=1S/C17H23IN4O4/c1-9(2)11-4-14(25-3)12(18)5-13(11)26-15-6-20-17(22-16(15)19)21-10(7-23)8-24/h4-6,9-10,23-24H,7-8H2,1-3H3,(H3,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X3 receptor expressed in rat C6-BU-1 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127833
BindingDB Entry DOI: 10.7270/Q2V40ZXF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at rat recombinant P2X3 receptor expressed in CHO cells by FLIPR assay |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 1
(RAT) | BDBM50118221

(9H-purine derivative | CHEMBL132722 | DIPHOSPHOMET...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H18N5O12P3/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(27-11)1-26-29(19,20)4-30(21,22)28-31(23,24)25/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,25)/t5-,7-,8-,11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA |
J Med Chem 48: 6887-96 (2005)
Article DOI: 10.1021/jm058018d
BindingDB Entry DOI: 10.7270/Q28C9X1X |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2X3 receptor expressed in HEK293-Tet-on cells assessed as alpha,beta-methylene-ATP-stimulated Ca2+ influx b... |
Bioorg Med Chem Lett 26: 3896-904 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.009
BindingDB Entry DOI: 10.7270/Q26W9FJD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2X3 receptor expressed in HEK293-Tet-on cells assessed as alpha,beta-methylene-ATP-stimulated Ca2+ influx b... |
Bioorg Med Chem Lett 26: 3896-904 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.009
BindingDB Entry DOI: 10.7270/Q26W9FJD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50499490
(CHEMBL3741992)Show SMILES Cc1cc(Cl)c(OCCO)cc1NC(=O)C1=CNC(=O)C[C@H]1c1ccc(F)cc1 |r,t:16| Show InChI InChI=1S/C21H20ClFN2O4/c1-12-8-17(22)19(29-7-6-26)10-18(12)25-21(28)16-11-24-20(27)9-15(16)13-2-4-14(23)5-3-13/h2-5,8,10-11,15,26H,6-7,9H2,1H3,(H,24,27)(H,25,28)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... |
J Med Chem 58: 8413-26 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00365
BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50415550
(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X2/3 receptor expressed in 1321n1c cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X2/3 receptor expressed in 1321n1c cells by FLIPR |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2/3
(Homo sapiens (Human)) | BDBM50257829

(2-(4-amino-5-(5-iodo-2-isopropyl-4-methoxyphenoxy)...)Show InChI InChI=1S/C17H23IN4O4/c1-9(2)11-4-14(25-3)12(18)5-13(11)26-15-6-20-17(22-16(15)19)21-10(7-23)8-24/h4-6,9-10,23-24H,7-8H2,1-3H3,(H3,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X2/3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127833
BindingDB Entry DOI: 10.7270/Q2V40ZXF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50499490

(CHEMBL3741992)Show SMILES Cc1cc(Cl)c(OCCO)cc1NC(=O)C1=CNC(=O)C[C@H]1c1ccc(F)cc1 |r,t:16| Show InChI InChI=1S/C21H20ClFN2O4/c1-12-8-17(22)19(29-7-6-26)10-18(12)25-21(28)16-11-24-20(27)9-15(16)13-2-4-14(23)5-3-13/h2-5,8,10-11,15,26H,6-7,9H2,1H3,(H,24,27)(H,25,28)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... |
J Med Chem 58: 8413-26 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00365
BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50257636

(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of P2X2 receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50415550

(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50415563
(CHEMBL605325)Show SMILES CCCc1nc(oc1C(=O)NC(C)CN1CCN(CC1)c1ncccn1)-c1ccccc1 Show InChI InChI=1S/C24H30N6O2/c1-3-8-20-21(32-23(28-20)19-9-5-4-6-10-19)22(31)27-18(2)17-29-13-15-30(16-14-29)24-25-11-7-12-26-24/h4-7,9-12,18H,3,8,13-17H2,1-2H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50415550

(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50596629
(CHEMBL5200337)Show SMILES COc1ccc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1OC |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 732 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 902 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 1
(RAT) | BDBM50540409
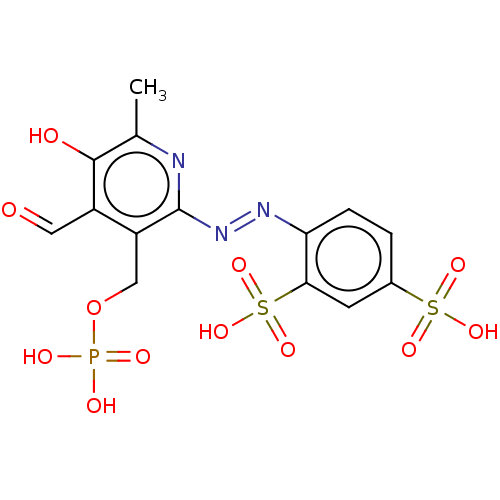
(CHEBI:34941 | CHEMBL69234)Show SMILES Cc1nc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O Show InChI InChI=1S/C14H14N3O12PS2/c1-7-13(19)9(5-18)10(6-29-30(20,21)22)14(15-7)17-16-11-3-2-8(31(23,24)25)4-12(11)32(26,27)28/h2-5,19H,6H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/b17-16+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X1 receptor |
J Med Chem 63: 6164-6178 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00435
BindingDB Entry DOI: 10.7270/Q24F1V93 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50596625
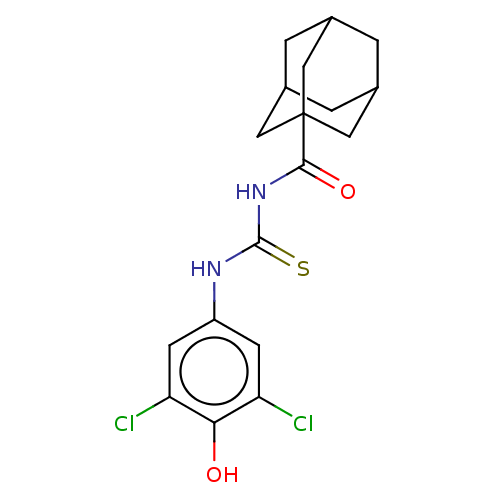
(CHEMBL5174645)Show SMILES Oc1c(Cl)cc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1Cl |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50596625

(CHEMBL5174645)Show SMILES Oc1c(Cl)cc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1Cl |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50596631
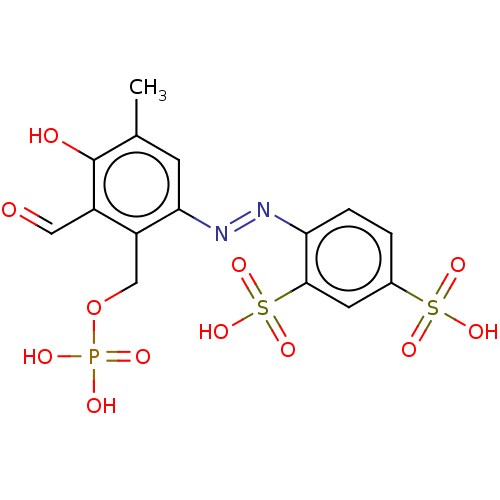
(CHEMBL1615626)Show SMILES Cc1cc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50596630

(CHEMBL5172961)Show SMILES Cc1ccc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)c(Br)c1 |TLB:18:17:20:14.13.12,18:13:20:17.19.16,THB:16:17:20.15.14:12,16:15:17.19.18:12| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50540409

(CHEBI:34941 | CHEMBL69234)Show SMILES Cc1nc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O Show InChI InChI=1S/C14H14N3O12PS2/c1-7-13(19)9(5-18)10(6-29-30(20,21)22)14(15-7)17-16-11-3-2-8(31(23,24)25)4-12(11)32(26,27)28/h2-5,19H,6H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/b17-16+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50415550

(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2X3 receptor expressed in HEK293-Tet-on cells assessed as alpha,beta-methylene-ATP-stimulated Ca2+ influx b... |
Bioorg Med Chem Lett 26: 3896-904 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.009
BindingDB Entry DOI: 10.7270/Q26W9FJD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50415550

(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant P2X3 receptor expressed in HEK293-Tet-on cells assessed as alpha,beta-methylene-ATP-stimulated Ca2+ influx b... |
Bioorg Med Chem Lett 26: 3896-904 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.009
BindingDB Entry DOI: 10.7270/Q26W9FJD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50596625

(CHEMBL5174645)Show SMILES Oc1c(Cl)cc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1Cl |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant phospho-MK2 (36-400) by HTRF assay |
Bioorg Med Chem Lett 20: 330-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.102
BindingDB Entry DOI: 10.7270/Q24M95GT |
More data for this
Ligand-Target Pair | |
Endoplasmin
(Canis familiaris) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(3-(6-amino-8-(6-iodobenzo[d][1,3]dioxol-5-ylthio)-9H-purin-9-yl)propyl)thioureido)-2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic a... |
J Med Chem 56: 6803-18 (2013)
Article DOI: 10.1021/jm400619b
BindingDB Entry DOI: 10.7270/Q2PN972K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase








