

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 155.1 BDBM50121955 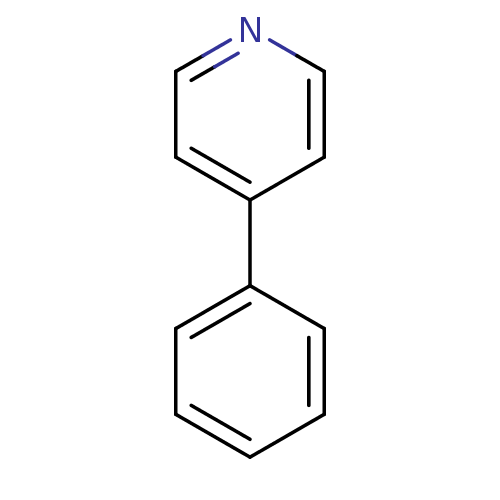 Purchase Purchase | Wt: 111.0 BDBM50543165  Purchase Purchase | Wt: 125.1 BDBM50543166 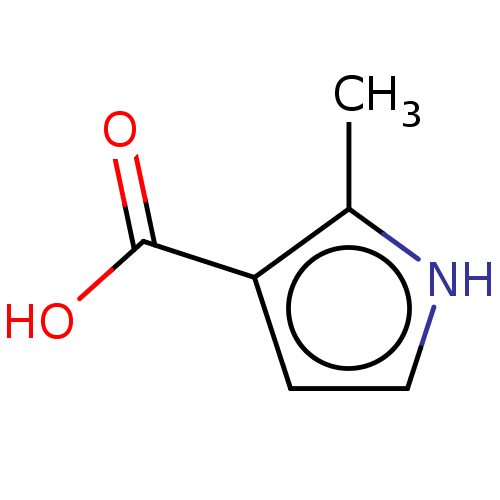 Purchase Purchase | Wt: 125.1 BDBM50543167  Purchase Purchase | Wt: 125.1 BDBM50543168 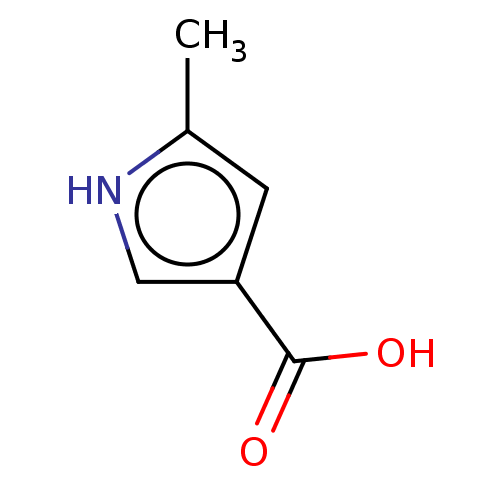 Purchase Purchase |
| Wt: 139.1 BDBM50543170  Purchase Purchase | Wt: 139.1 BDBM50543171 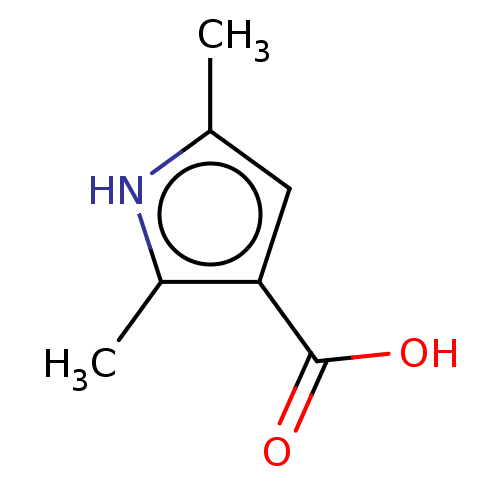 Purchase Purchase | Wt: 153.1 BDBM50543172  Purchase Purchase | Wt: 162.1 BDBM600881  | Wt: 160.1 BDBM600887  |
| Wt: 159.1 BDBM600840  | Wt: 146.1 BDBM600902  | Wt: 161.1 BDBM600903  Purchase Purchase | Wt: 161.1 BDBM600905  | Wt: 146.1 BDBM600907  |
| Displayed 1 to 15 (of 606 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600840 (1-(p-tolyl)triazole | US11634391, Compound 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600887 (2-(p-tolyl)-1,3,4-oxadiazole | US11634391, Compoun...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600907 (2-phenyl-1,3,4-oxadiazole | US11634391, Compound 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600881 (5-phenyl-1,3,4-oxadiazol-2-ol | US11634391, Compou...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600902 (1-phenyl-1H-tetrazole | US11634391, Compound 134) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600903 (5-phenyl-1,3,4-oxadiazol-2-amine | US11634391, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM600905 (3-phenyl-1,2,4-oxadiazol-5-amine | US11634391, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZS31FM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543172 (CHEMBL4636951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543171 (CHEMBL4645573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543168 (CHEMBL247338) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543167 (CHEMBL4649435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543166 (CHEMBL4636656) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543165 (CHEBI:68076 | CHEMBL79155) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50543170 (CHEMBL4646068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human Notum (S81 to T451 residues) Cys330Ser mutant expressed in HEK293S GnTI cells using OPTS as substrate incubated for 40 mins by fl... | J Med Chem 63: 9464-9483 (2020) Article DOI: 10.1021/acs.jmedchem.0c00660 BindingDB Entry DOI: 10.7270/Q24J0JNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of native signal containing Klebsiella pneumoniae OXA-48 using nitrocefin substrate pre-incubated for 5 mins before substrate addition | J Med Chem 59: 5542-54 (2016) Article DOI: 10.1021/acs.jmedchem.6b00660 BindingDB Entry DOI: 10.7270/Q2NP26CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Binding affinity to native signal deficient and TEV cleavage site containing His-tagged Klebsiella pneumoniae OXA-48 expressed in Escherichia coli as... | J Med Chem 59: 5542-54 (2016) Article DOI: 10.1021/acs.jmedchem.6b00660 BindingDB Entry DOI: 10.7270/Q2NP26CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Z£rich Curated by ChEMBL | Assay Description Binding affinity to recombinant GST-tagged human BRPF1 expressed in Escherichia coli BL21 (DE3) after 1 hr by qPCR-based BromoScan assay | J Med Chem 59: 5555-61 (2016) Article DOI: 10.1021/acs.jmedchem.6b00215 BindingDB Entry DOI: 10.7270/Q22Z17GF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate | J Med Chem 45: 5628-39 (2002) BindingDB Entry DOI: 10.7270/Q20C4V3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50121955 (4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid | J Med Chem 45: 5628-39 (2002) BindingDB Entry DOI: 10.7270/Q20C4V3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||