 Found 60 hits in this display
Found 60 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activity |
Antimicrob Agents Chemother 52: 1302-17 (2008)
Article DOI: 10.1128/AAC.01324-07
BindingDB Entry DOI: 10.7270/Q21836R5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339126
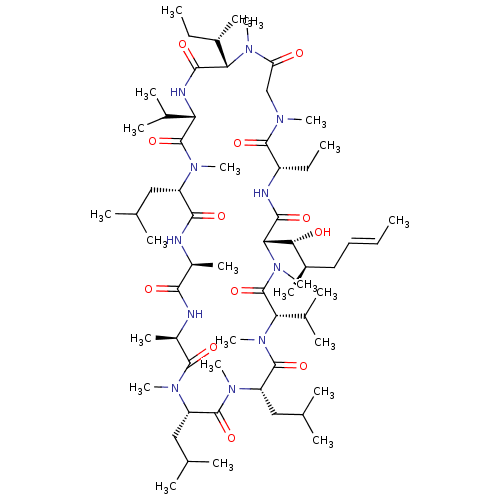
((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-24-sec-buty...)Show SMILES CC[C@H](C)[C@@H]1N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-28-29-40(15)52(75)51-56(79)65-43(27-3)58(81)67(18)33-47(74)71(22)50(39(14)26-2)55(78)66-48(37(10)11)61(84)68(19)44(30-34(4)5)54(77)63-41(16)53(76)64-42(17)57(80)69(20)45(31-35(6)7)59(82)70(21)46(32-36(8)9)60(83)72(23)49(38(12)13)62(85)73(51)24/h25,28,34-46,48-52,75H,26-27,29-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b28-25+/t39-,40+,41-,42+,43-,44-,45-,46-,48-,49-,50-,51-,52+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activity |
Antimicrob Agents Chemother 52: 1302-17 (2008)
Article DOI: 10.1128/AAC.01324-07
BindingDB Entry DOI: 10.7270/Q21836R5 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030555
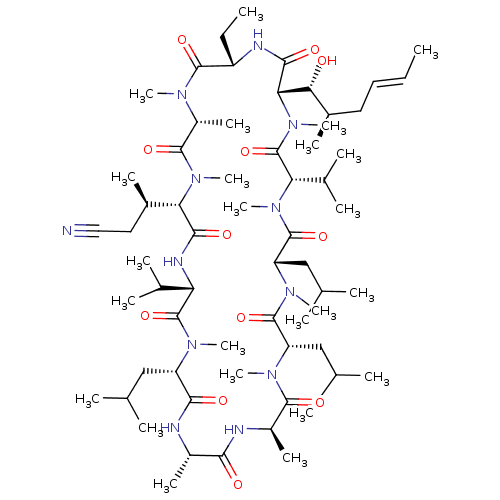
(CHEMBL3344493 | US9566312, Compound 2.10)Show SMILES [H][C@@]1([C@H](C)CC#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H110N12O12/c1-25-27-28-40(14)52(76)51-56(80)67-44(26-2)59(83)69(18)43(17)58(82)74(23)50(39(13)29-30-64)55(79)68-48(37(9)10)62(86)70(19)45(31-34(3)4)54(78)65-41(15)53(77)66-42(16)57(81)71(20)46(32-35(5)6)60(84)72(21)47(33-36(7)8)61(85)73(22)49(38(11)12)63(87)75(51)24/h25,27,34-52,76H,26,28-29,31-33H2,1-24H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b27-25+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030554
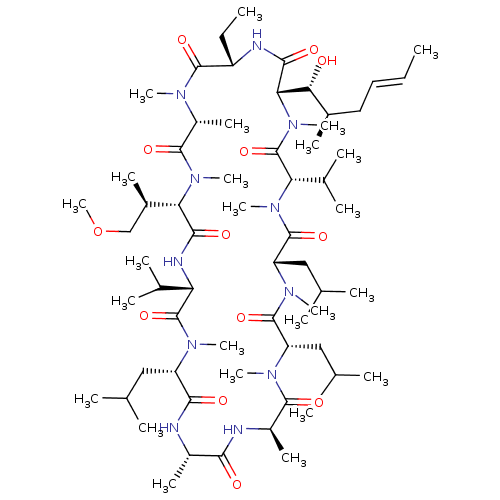
(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286508
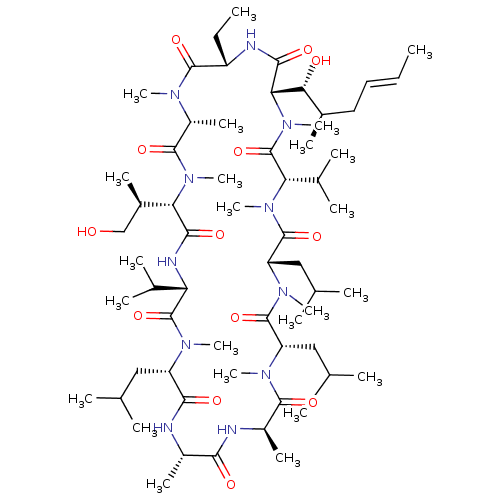
(US9566312, Compound 2.5.1)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CO)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O13/c1-25-27-28-38(13)51(75)50-55(79)65-43(26-2)58(82)67(18)42(17)57(81)72(23)49(39(14)32-74)54(78)66-47(36(9)10)61(85)68(19)44(29-33(3)4)53(77)63-40(15)52(76)64-41(16)56(80)69(20)45(30-34(5)6)59(83)70(21)46(31-35(7)8)60(84)71(22)48(37(11)12)62(86)73(50)24/h25,27,33-51,74-75H,26,28-32H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t38-,39-,40+,41-,42-,43+,44+,45+,46+,47+,48+,49+,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50030555

(CHEMBL3344493 | US9566312, Compound 2.10)Show SMILES [H][C@@]1([C@H](C)CC#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H110N12O12/c1-25-27-28-40(14)52(76)51-56(80)67-44(26-2)59(83)69(18)43(17)58(82)74(23)50(39(13)29-30-64)55(79)68-48(37(9)10)62(86)70(19)45(31-34(3)4)54(78)65-41(15)53(77)66-42(16)57(81)71(20)46(32-35(5)6)60(84)72(21)47(33-36(7)8)61(85)73(22)49(38(11)12)63(87)75(51)24/h25,27,34-52,76H,26,28-29,31-33H2,1-24H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b27-25+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50030555

(CHEMBL3344493 | US9566312, Compound 2.10)Show SMILES [H][C@@]1([C@H](C)CC#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H110N12O12/c1-25-27-28-40(14)52(76)51-56(80)67-44(26-2)59(83)69(18)43(17)58(82)74(23)50(39(13)29-30-64)55(79)68-48(37(9)10)62(86)70(19)45(31-34(3)4)54(78)65-41(15)53(77)66-42(16)57(81)71(20)46(32-35(5)6)60(84)72(21)47(33-36(7)8)61(85)73(22)49(38(11)12)63(87)75(51)24/h25,27,34-52,76H,26,28-29,31-33H2,1-24H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b27-25+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM50030555

(CHEMBL3344493 | US9566312, Compound 2.10)Show SMILES [H][C@@]1([C@H](C)CC#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H110N12O12/c1-25-27-28-40(14)52(76)51-56(80)67-44(26-2)59(83)69(18)43(17)58(82)74(23)50(39(13)29-30-64)55(79)68-48(37(9)10)62(86)70(19)45(31-34(3)4)54(78)65-41(15)53(77)66-42(16)57(81)71(20)46(32-35(5)6)60(84)72(21)47(33-36(7)8)61(85)73(22)49(38(11)12)63(87)75(51)24/h25,27,34-52,76H,26,28-29,31-33H2,1-24H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b27-25+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286575
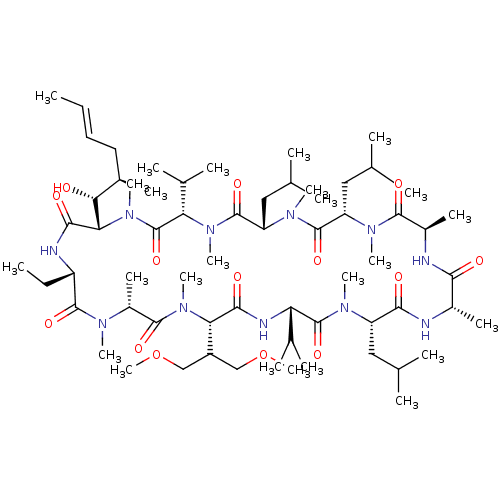
(US9566312, Compound 2.11)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(COC)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O14/c1-26-28-29-40(13)53(76)52-57(80)67-45(27-2)60(83)69(17)43(16)59(82)74(22)51(44(33-88-24)34-89-25)56(79)68-49(38(9)10)63(86)70(18)46(30-35(3)4)55(78)65-41(14)54(77)66-42(15)58(81)71(19)47(31-36(5)6)61(84)72(20)48(32-37(7)8)62(85)73(21)50(39(11)12)64(87)75(52)23/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41+,42-,43-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286575

(US9566312, Compound 2.11)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(COC)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O14/c1-26-28-29-40(13)53(76)52-57(80)67-45(27-2)60(83)69(17)43(16)59(82)74(22)51(44(33-88-24)34-89-25)56(79)68-49(38(9)10)63(86)70(18)46(30-35(3)4)55(78)65-41(14)54(77)66-42(15)58(81)71(19)47(31-36(5)6)61(84)72(20)48(32-37(7)8)62(85)73(21)50(39(11)12)64(87)75(52)23/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41+,42-,43-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286575

(US9566312, Compound 2.11)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(COC)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O14/c1-26-28-29-40(13)53(76)52-57(80)67-45(27-2)60(83)69(17)43(16)59(82)74(22)51(44(33-88-24)34-89-25)56(79)68-49(38(9)10)63(86)70(18)46(30-35(3)4)55(78)65-41(14)54(77)66-42(15)58(81)71(19)47(31-36(5)6)61(84)72(20)48(32-37(7)8)62(85)73(21)50(39(11)12)64(87)75(52)23/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41+,42-,43-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286576

(US9566312, Compound 2.12)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCC(C)(C)O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H119N11O13/c1-27-29-30-42(14)54(78)53-58(82)69-46(28-2)61(85)71(20)45(17)60(84)76(25)52(41(13)31-32-66(18,19)90)57(81)70-50(39(9)10)64(88)72(21)47(33-36(3)4)56(80)67-43(15)55(79)68-44(16)59(83)73(22)48(34-37(5)6)62(86)74(23)49(35-38(7)8)63(87)75(24)51(40(11)12)65(89)77(53)26/h27,29,36-54,78,90H,28,30-35H2,1-26H3,(H,67,80)(H,68,79)(H,69,82)(H,70,81)/b29-27+/t41-,42-,43+,44-,45-,46+,47+,48+,49+,50+,51+,52+,53+,54-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286576

(US9566312, Compound 2.12)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCC(C)(C)O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H119N11O13/c1-27-29-30-42(14)54(78)53-58(82)69-46(28-2)61(85)71(20)45(17)60(84)76(25)52(41(13)31-32-66(18,19)90)57(81)70-50(39(9)10)64(88)72(21)47(33-36(3)4)56(80)67-43(15)55(79)68-44(16)59(83)73(22)48(34-37(5)6)62(86)74(23)49(35-38(7)8)63(87)75(24)51(40(11)12)65(89)77(53)26/h27,29,36-54,78,90H,28,30-35H2,1-26H3,(H,67,80)(H,68,79)(H,69,82)(H,70,81)/b29-27+/t41-,42-,43+,44-,45-,46+,47+,48+,49+,50+,51+,52+,53+,54-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286576

(US9566312, Compound 2.12)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCC(C)(C)O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H119N11O13/c1-27-29-30-42(14)54(78)53-58(82)69-46(28-2)61(85)71(20)45(17)60(84)76(25)52(41(13)31-32-66(18,19)90)57(81)70-50(39(9)10)64(88)72(21)47(33-36(3)4)56(80)67-43(15)55(79)68-44(16)59(83)73(22)48(34-37(5)6)62(86)74(23)49(35-38(7)8)63(87)75(24)51(40(11)12)65(89)77(53)26/h27,29,36-54,78,90H,28,30-35H2,1-26H3,(H,67,80)(H,68,79)(H,69,82)(H,70,81)/b29-27+/t41-,42-,43+,44-,45-,46+,47+,48+,49+,50+,51+,52+,53+,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286578

(US9566312, Compound 2.13)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCOC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-28-29-41(14)53(76)52-57(80)67-45(27-2)60(83)69(18)44(17)59(82)74(23)51(40(13)30-31-88-25)56(79)68-49(38(9)10)63(86)70(19)46(32-35(3)4)55(78)65-42(15)54(77)66-43(16)58(81)71(20)47(33-36(5)6)61(84)72(21)48(34-37(7)8)62(85)73(22)50(39(11)12)64(87)75(52)24/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286578

(US9566312, Compound 2.13)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCOC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-28-29-41(14)53(76)52-57(80)67-45(27-2)60(83)69(18)44(17)59(82)74(23)51(40(13)30-31-88-25)56(79)68-49(38(9)10)63(86)70(19)46(32-35(3)4)55(78)65-42(15)54(77)66-43(16)58(81)71(20)47(33-36(5)6)61(84)72(21)48(34-37(7)8)62(85)73(22)50(39(11)12)64(87)75(52)24/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286578

(US9566312, Compound 2.13)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CCOC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-28-29-41(14)53(76)52-57(80)67-45(27-2)60(83)69(18)44(17)59(82)74(23)51(40(13)30-31-88-25)56(79)68-49(38(9)10)63(86)70(19)46(32-35(3)4)55(78)65-42(15)54(77)66-43(16)58(81)71(20)47(33-36(5)6)61(84)72(21)48(34-37(7)8)62(85)73(22)50(39(11)12)64(87)75(52)24/h26,28,35-53,76H,27,29-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-26+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286619
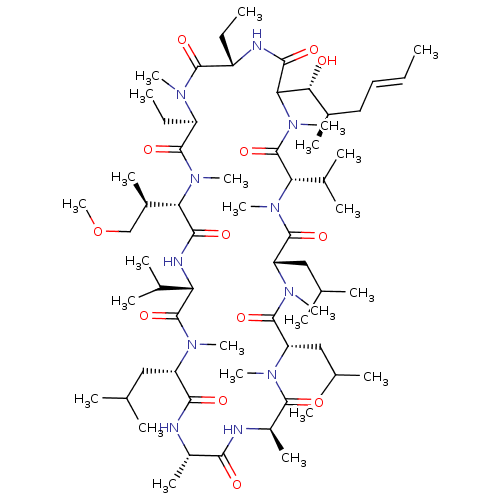
(US9566312, Compound 3.1)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CC)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-29-30-40(14)53(76)52-57(80)67-44(27-2)59(82)69(18)45(28-3)60(83)74(23)51(41(15)34-88-25)56(79)68-49(38(10)11)63(86)70(19)46(31-35(4)5)55(78)65-42(16)54(77)66-43(17)58(81)71(20)47(32-36(6)7)61(84)72(21)48(33-37(8)9)62(85)73(22)50(39(12)13)64(87)75(52)24/h26,29,35-53,76H,27-28,30-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b29-26+/t40-,41-,42+,43-,44+,45-,46+,47+,48+,49+,50+,51+,52?,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286619

(US9566312, Compound 3.1)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CC)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-29-30-40(14)53(76)52-57(80)67-44(27-2)59(82)69(18)45(28-3)60(83)74(23)51(41(15)34-88-25)56(79)68-49(38(10)11)63(86)70(19)46(31-35(4)5)55(78)65-42(16)54(77)66-43(17)58(81)71(20)47(32-36(6)7)61(84)72(21)48(33-37(8)9)62(85)73(22)50(39(12)13)64(87)75(52)24/h26,29,35-53,76H,27-28,30-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b29-26+/t40-,41-,42+,43-,44+,45-,46+,47+,48+,49+,50+,51+,52?,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286619

(US9566312, Compound 3.1)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CC)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C64H115N11O13/c1-26-29-30-40(14)53(76)52-57(80)67-44(27-2)59(82)69(18)45(28-3)60(83)74(23)51(41(15)34-88-25)56(79)68-49(38(10)11)63(86)70(19)46(31-35(4)5)55(78)65-42(16)54(77)66-43(17)58(81)71(20)47(32-36(6)7)61(84)72(21)48(33-37(8)9)62(85)73(22)50(39(12)13)64(87)75(52)24/h26,29,35-53,76H,27-28,30-34H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b29-26+/t40-,41-,42+,43-,44+,45-,46+,47+,48+,49+,50+,51+,52?,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286622

(US9566312, Compound 3.2.3)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CO)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14/c1-25-27-28-39(13)52(76)51-56(80)66-43(26-2)58(82)71(20)47(32-75)61(85)73(22)50(40(14)33-88-24)55(79)67-48(37(9)10)62(86)68(17)44(29-34(3)4)54(78)64-41(15)53(77)65-42(16)57(81)69(18)45(30-35(5)6)59(83)70(19)46(31-36(7)8)60(84)72(21)49(38(11)12)63(87)74(51)23/h25,27,34-52,75-76H,26,28-33H2,1-24H3,(H,64,78)(H,65,77)(H,66,80)(H,67,79)/b27-25+/t39-,40-,41+,42-,43+,44+,45+,46+,47-,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286622

(US9566312, Compound 3.2.3)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CO)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14/c1-25-27-28-39(13)52(76)51-56(80)66-43(26-2)58(82)71(20)47(32-75)61(85)73(22)50(40(14)33-88-24)55(79)67-48(37(9)10)62(86)68(17)44(29-34(3)4)54(78)64-41(15)53(77)65-42(16)57(81)69(18)45(30-35(5)6)59(83)70(19)46(31-36(7)8)60(84)72(21)49(38(11)12)63(87)74(51)23/h25,27,34-52,75-76H,26,28-33H2,1-24H3,(H,64,78)(H,65,77)(H,66,80)(H,67,79)/b27-25+/t39-,40-,41+,42-,43+,44+,45+,46+,47-,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286622

(US9566312, Compound 3.2.3)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](CO)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14/c1-25-27-28-39(13)52(76)51-56(80)66-43(26-2)58(82)71(20)47(32-75)61(85)73(22)50(40(14)33-88-24)55(79)67-48(37(9)10)62(86)68(17)44(29-34(3)4)54(78)64-41(15)53(77)65-42(16)57(81)69(18)45(30-35(5)6)59(83)70(19)46(31-36(7)8)60(84)72(21)49(38(11)12)63(87)74(51)23/h25,27,34-52,75-76H,26,28-33H2,1-24H3,(H,64,78)(H,65,77)(H,66,80)(H,67,79)/b27-25+/t39-,40-,41+,42-,43+,44+,45+,46+,47-,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286623
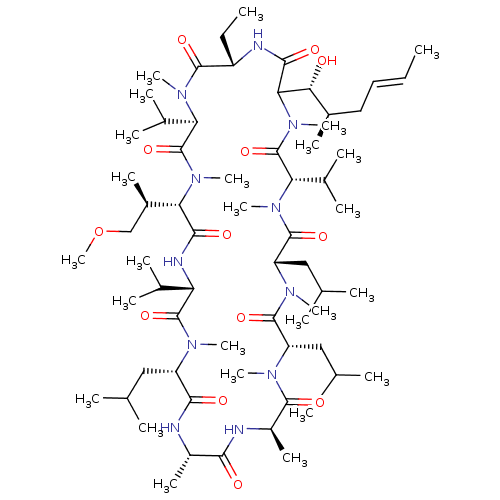
(US9566312, Compound 3.3)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](C(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C65H117N11O13/c1-27-29-30-41(15)54(77)53-58(81)68-45(28-2)60(83)73(22)50(39(11)12)64(87)75(24)52(42(16)34-89-26)57(80)69-49(38(9)10)63(86)70(19)46(31-35(3)4)56(79)66-43(17)55(78)67-44(18)59(82)71(20)47(32-36(5)6)61(84)72(21)48(33-37(7)8)62(85)74(23)51(40(13)14)65(88)76(53)25/h27,29,35-54,77H,28,30-34H2,1-26H3,(H,66,79)(H,67,78)(H,68,81)(H,69,80)/b29-27+/t41-,42-,43+,44-,45+,46+,47+,48+,49+,50-,51+,52+,53?,54-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 13.7 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286623

(US9566312, Compound 3.3)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](C(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C65H117N11O13/c1-27-29-30-41(15)54(77)53-58(81)68-45(28-2)60(83)73(22)50(39(11)12)64(87)75(24)52(42(16)34-89-26)57(80)69-49(38(9)10)63(86)70(19)46(31-35(3)4)56(79)66-43(17)55(78)67-44(18)59(82)71(20)47(32-36(5)6)61(84)72(21)48(33-37(7)8)62(85)74(23)51(40(13)14)65(88)76(53)25/h27,29,35-54,77H,28,30-34H2,1-26H3,(H,66,79)(H,67,78)(H,68,81)(H,69,80)/b29-27+/t41-,42-,43+,44-,45+,46+,47+,48+,49+,50-,51+,52+,53?,54-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286623

(US9566312, Compound 3.3)Show SMILES CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)COC)N(C)C(=O)[C@@H](C(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C65H117N11O13/c1-27-29-30-41(15)54(77)53-58(81)68-45(28-2)60(83)73(22)50(39(11)12)64(87)75(24)52(42(16)34-89-26)57(80)69-49(38(9)10)63(86)70(19)46(31-35(3)4)56(79)66-43(17)55(78)67-44(18)59(82)71(20)47(32-36(5)6)61(84)72(21)48(33-37(7)8)62(85)74(23)51(40(13)14)65(88)76(53)25/h27,29,35-54,77H,28,30-34H2,1-26H3,(H,66,79)(H,67,78)(H,68,81)(H,69,80)/b29-27+/t41-,42-,43+,44-,45+,46+,47+,48+,49+,50-,51+,52+,53?,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 2.05 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 1.45 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 12.7 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
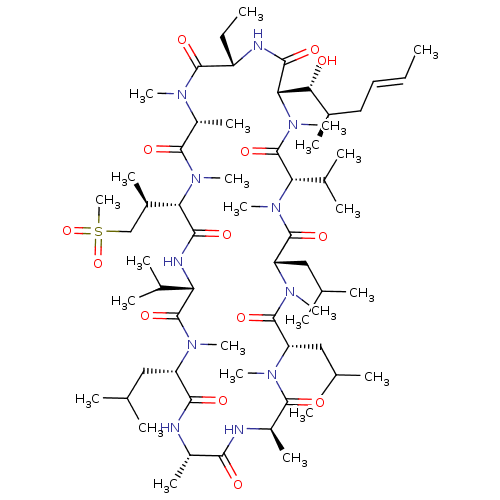
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286507
(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286507

(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286507

(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286508

(US9566312, Compound 2.5.1)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CO)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O13/c1-25-27-28-38(13)51(75)50-55(79)65-43(26-2)58(82)67(18)42(17)57(81)72(23)49(39(14)32-74)54(78)66-47(36(9)10)61(85)68(19)44(29-33(3)4)53(77)63-40(15)52(76)64-41(16)56(80)69(20)45(30-34(5)6)59(83)70(21)46(31-35(7)8)60(84)71(22)48(37(11)12)62(86)73(50)24/h25,27,33-51,74-75H,26,28-32H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t38-,39-,40+,41-,42-,43+,44+,45+,46+,47+,48+,49+,50+,51-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by fluorescence polarization assay |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NS5B S282T mutant in HCV genotype 1b infected in HuH7.5Lcu-Neo cells assessed as reduction in viral replication by luciferase reporter ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2BR8WV9 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286508

(US9566312, Compound 2.5.1)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CO)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O13/c1-25-27-28-38(13)51(75)50-55(79)65-43(26-2)58(82)67(18)42(17)57(81)72(23)49(39(14)32-74)54(78)66-47(36(9)10)61(85)68(19)44(29-33(3)4)53(77)63-40(15)52(76)64-41(16)56(80)69(20)45(30-34(5)6)59(83)70(21)46(31-35(7)8)60(84)71(22)48(37(11)12)62(86)73(50)24/h25,27,33-51,74-75H,26,28-32H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t38-,39-,40+,41-,42-,43+,44+,45+,46+,47+,48+,49+,50+,51-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM50044551

(CHEMBL3327189)Show SMILES [H][C@@]1([#6@H](-[#8])-[#6@H](-[#6])-[#6]\[#6]=[#6]\[#6])[#7](-[#6])-[#6](=O)-[#6@H](-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#6](-[#6])-[#6])-[#7](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#6])-[#7]-[#6]1=O |r| Show InChI InChI=1S/C68H121N11O12/c1-28-30-31-45(17)57(80)56-60(83)71-49(29-2)63(86)72(21)48(20)62(85)75(24)53(37-42(11)12)66(89)79(33-32-38(3)4)55(44(15)16)68(91)73(22)50(34-39(5)6)59(82)69-46(18)58(81)70-47(19)61(84)74(23)51(35-40(7)8)64(87)76(25)52(36-41(9)10)65(88)77(26)54(43(13)14)67(90)78(56)27/h28,30,32,39-57,80H,29,31,33-37H2,1-27H3,(H,69,82)(H,70,81)(H,71,83)/b30-28+/t45-,46+,47-,48+,49+,50+,51+,52+,53+,54+,55+,56+,57-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to cyclophilin D (unknown origin) |
J Med Chem 57: 7145-59 (2014)
Article DOI: 10.1021/jm500223x
BindingDB Entry DOI: 10.7270/Q25T3N47 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase





























































