

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50247011 (CHEMBL4080419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50247011 (CHEMBL4080419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50247022 (CHEMBL4089230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246979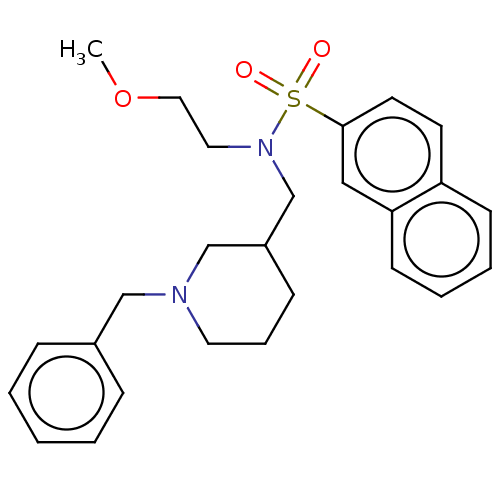 (CHEMBL4105611 | US20230331674, Table 1.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of AChE in mouse brain homogenate using acetylthiocholine as substrate preincubated for 300 secs followed by substrate addition by Ellman'... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50247010 (CHEMBL4068039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50027376 (CHEMBL3338391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246974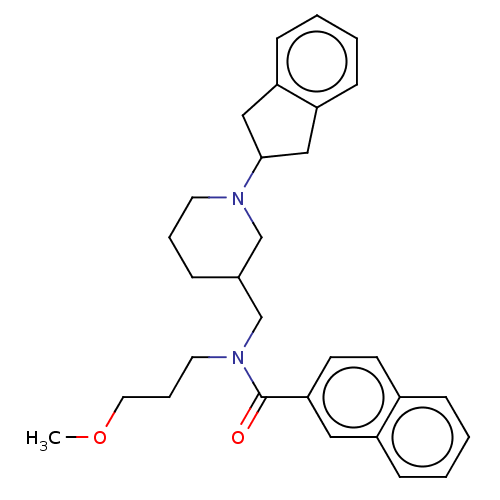 (CHEMBL4097939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246977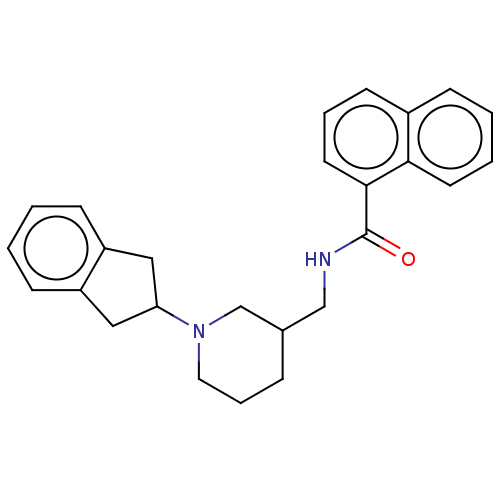 (CHEMBL4077192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246976 (CHEMBL4060562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine as substrate after 1 min by Ellman's method | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246978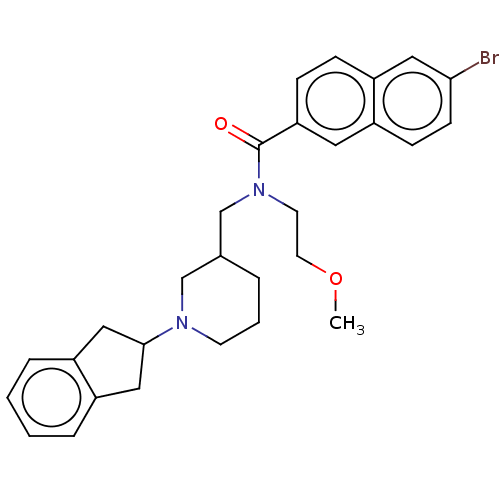 (CHEMBL4065481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246975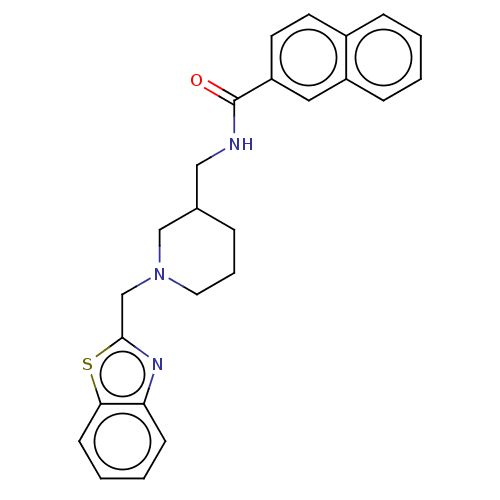 (CHEMBL4078849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 632 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50246973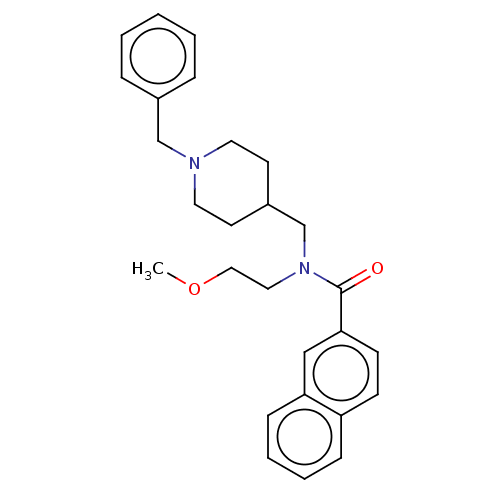 (CHEMBL4077386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 702 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50247009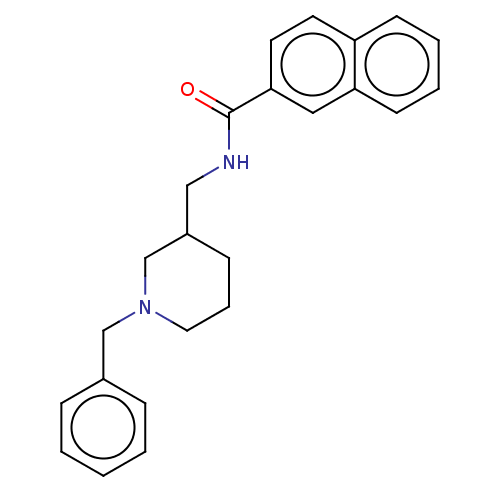 (CHEMBL4075840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate after 1 min by Ellman's method | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Mus musculus (Mouse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of BuChE in mouse brain homogenate using butyrylthiocholine as substrate preincubated for 300 secs followed by substrate addition by Ellma... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50247022 (CHEMBL4089230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50247009 (CHEMBL4075840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246974 (CHEMBL4097939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246975 (CHEMBL4078849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246976 (CHEMBL4060562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246977 (CHEMBL4077192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246978 (CHEMBL4065481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246979 (CHEMBL4105611 | US20230331674, Table 1.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50246973 (CHEMBL4077386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50027376 (CHEMBL3338391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse AChE using acetylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured for 1 min by E... | J Med Chem 61: 119-139 (2018) Article DOI: 10.1021/acs.jmedchem.7b01086 BindingDB Entry DOI: 10.7270/Q2S75JRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||