

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50318031 (CHEMBL1097279 | cangrelor) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor by [35S]GTPgammaS binding assay | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318031 (CHEMBL1097279 | cangrelor) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318030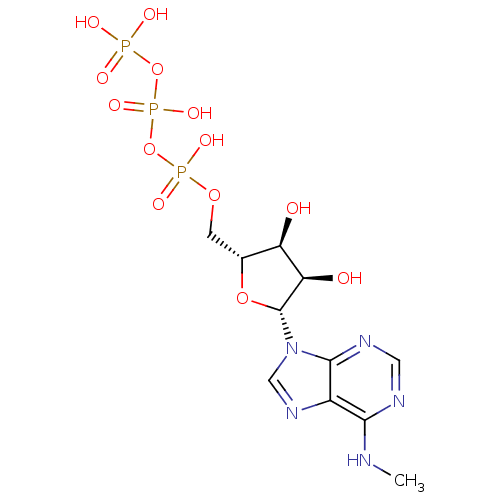 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50318029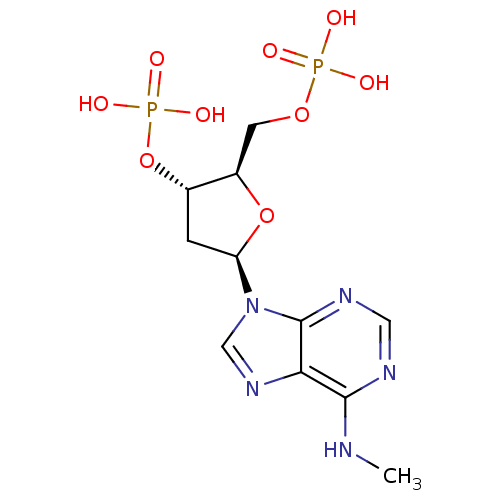 (CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 508 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor by [35S]GTPgammaS binding assay | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318029 (CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 508 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318028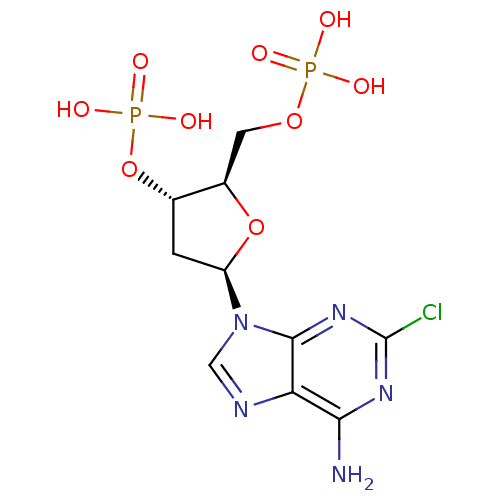 (CHEMBL1094760 | diammonium (2R,3S,5R)-5-(6-amino-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 582 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002313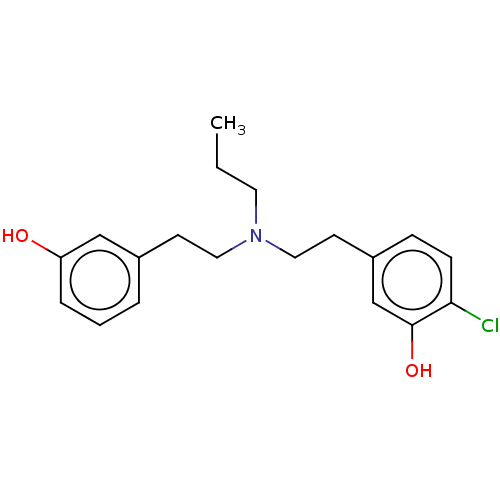 (2-Chloro-5-(2-{[2-(3-hydroxy-phenyl)-ethyl]-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002310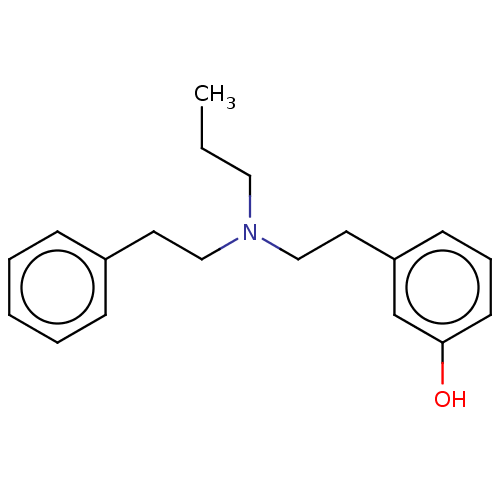 (3-[2-(Phenethyl-propyl-amino)-ethyl]-phenol; hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002314 (2-Chloro-5-(2-{[3-(4-hydroxy-phenyl)-propyl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002309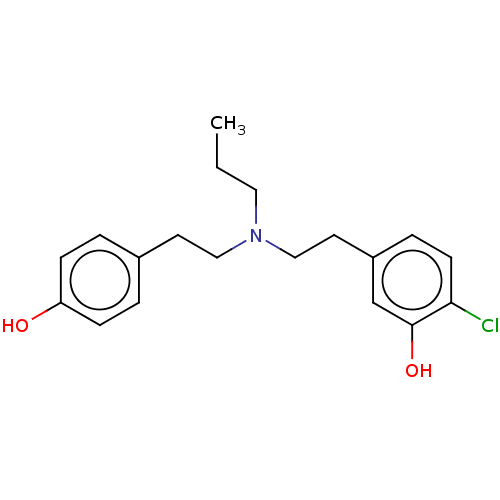 (2-Chloro-5-(2-{[2-(4-hydroxy-phenyl)-ethyl]-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002311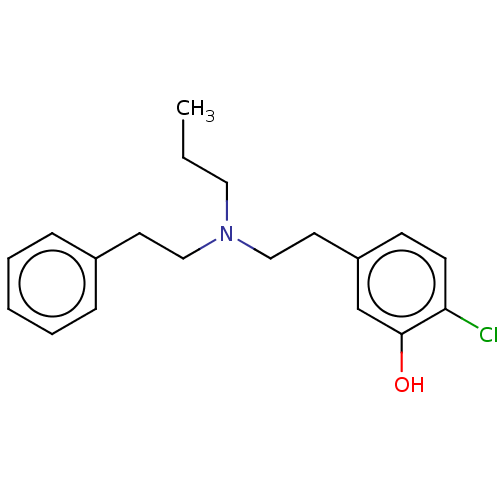 (2-Chloro-5-[2-(phenethyl-propyl-amino)-ethyl]-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002313 (2-Chloro-5-(2-{[2-(3-hydroxy-phenyl)-ethyl]-propyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002312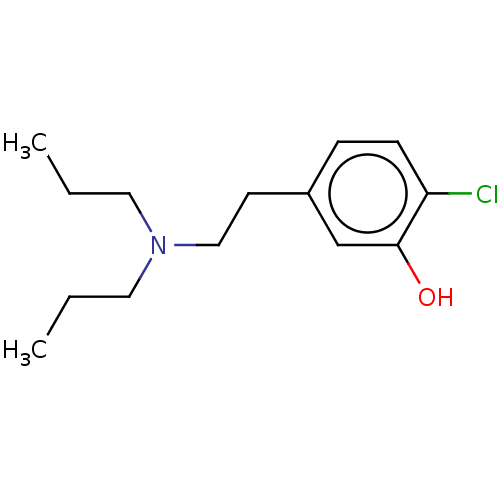 (2-Chloro-5-(2-dipropylamino-ethyl)-phenol; hydrobr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002314 (2-Chloro-5-(2-{[3-(4-hydroxy-phenyl)-propyl]-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002309 (2-Chloro-5-(2-{[2-(4-hydroxy-phenyl)-ethyl]-propyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002311 (2-Chloro-5-[2-(phenethyl-propyl-amino)-ethyl]-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002315 (5-(2-Amino-ethyl)-2-chloro-phenol; hydrobromide | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002315 (5-(2-Amino-ethyl)-2-chloro-phenol; hydrobromide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]spiperone in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002312 (2-Chloro-5-(2-dipropylamino-ethyl)-phenol; hydrobr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002310 (3-[2-(Phenethyl-propyl-amino)-ethyl]-phenol; hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibitory activity against [3H]-SCH- 23390 in Rat Striatal membrane | J Med Chem 35: 4408-14 (1992) BindingDB Entry DOI: 10.7270/Q2WH2QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318024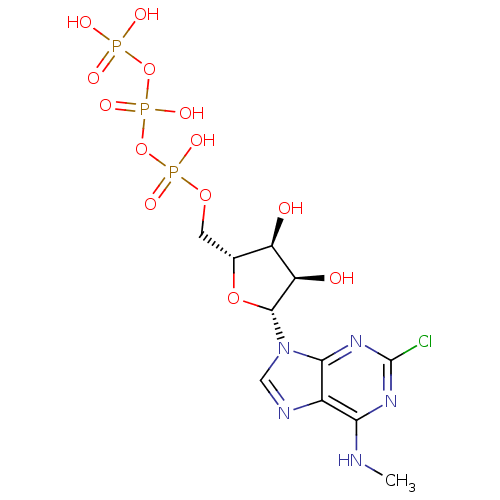 (((2R,3S,4R,5R)-5-(2-chloro-6-(methylamino)-9H-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318022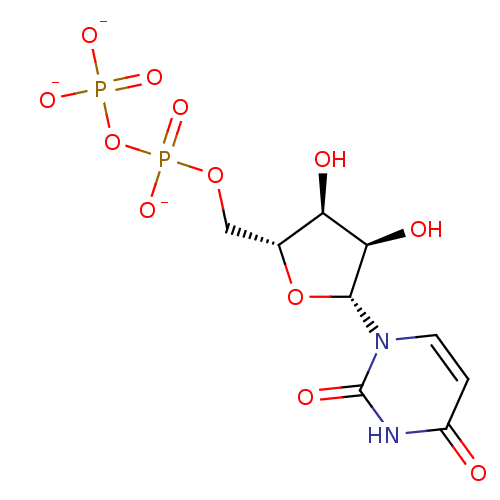 (CHEMBL1096401 | uridine diphosphate trisodium salt) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318025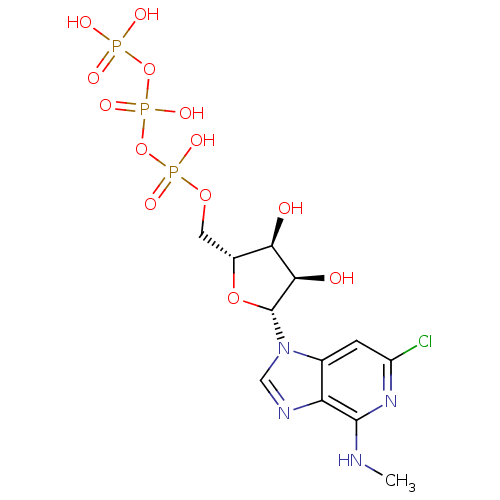 (((2R,3S,4R,5R)-5-(6-chloro-4-(methylamino)-1H-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318026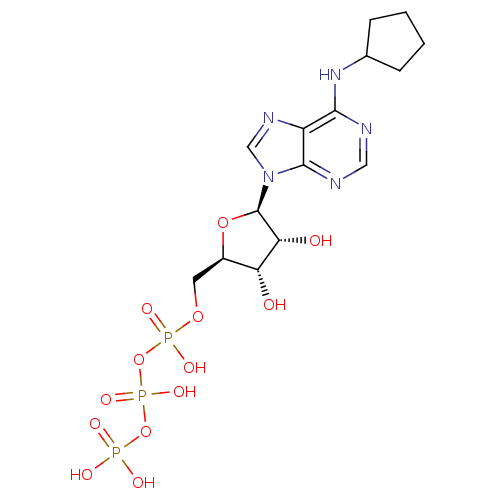 (((2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318027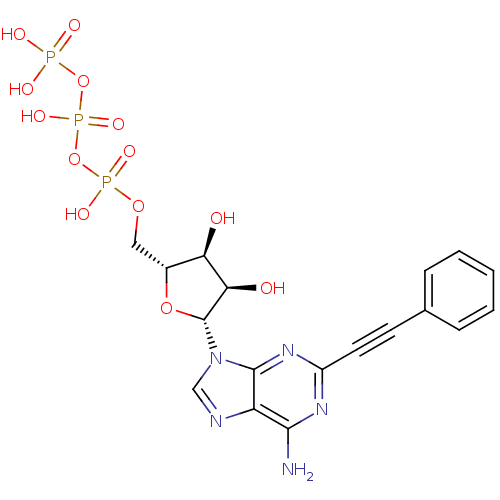 (((2R,3S,4R,5R)-5-(6-amino-2-(phenylethynyl)-9H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50306710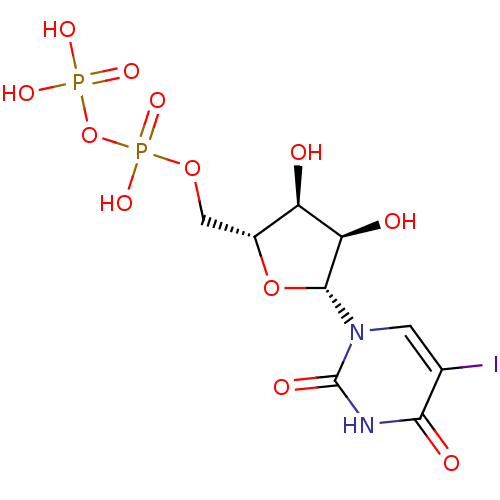 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 945 | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Agonist activity at human GPR17 expressed in human 1321N1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by rapid filtration ass... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||