 Found 147 hits with Last Name = 'abdel-hamid' and Initial = 'mk'
Found 147 hits with Last Name = 'abdel-hamid' and Initial = 'mk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50128358

(CHEMBL3629347)Show SMILES CCCCC(NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52?,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant melanocortin 4 receptor expressed in CHO cells |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176065
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-DAMP from human recombinant Muscarinic acetylcholine receptor M3 expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl-spiperone from human recombinant D2S receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]U 69593 from rat recombinant kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM28582
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [125I](+/-)DOI from human recombinant 5-HT2B receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50233962
(CHEMBL4068783)Show SMILES CCCCC(NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(O)=O |r| Show InChI InChI=1S/C35H55N7O11/c1-6-7-13-23(35(52)53)39-31(48)24(14-19(2)3)38-27(44)17-37-34(51)29(20(4)5)42-32(49)25(15-21-11-9-8-10-12-21)40-33(50)26(18-43)41-30(47)22(36)16-28(45)46/h8-12,19-20,22-26,29,43H,6-7,13-18,36H2,1-5H3,(H,37,51)(H,38,44)(H,39,48)(H,40,50)(H,41,47)(H,42,49)(H,45,46)(H,52,53)/t22-,23?,24-,25-,26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human recombinant tachykinin NK2 receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human recombinant norepinephrine transporter expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50005534
(1-(1-(benzo[b]thiophen-2-yl)cyclohexyl)piperidine ...)Show InChI InChI=1S/C19H25NS/c1-5-11-19(12-6-1,20-13-7-2-8-14-20)18-15-16-9-3-4-10-17(16)21-18/h3-4,9-10,15H,1-2,5-8,11-14H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from human recombinant dopamine transporter expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]pirenzepine from human recombinant Muscarinic acetylcholine receptor M1 expressed in CHO cells after 60 mins by scintillation cou... |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10868
(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10868

(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT5a receptor in HEK293 cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM181119
(US9138393, Cimetidine | US9144538, Cimetidine)Show InChI InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221270

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)C(=NNC(=O)Nc3nnc(S)s3)c2c1 |w:11.11| Show InChI InChI=1S/C11H7N7O4S2/c19-8-7(5-3-4(18(21)22)1-2-6(5)12-8)14-15-9(20)13-10-16-17-11(23)24-10/h1-3H,(H,17,23)(H,12,14,19)(H2,13,15,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221273
(4-(4,5-dyl)-1-[alpha-(3-pyridyl)methylene]semicarb...)Show InChI InChI=1S/C9H8N6OS2/c16-7(12-8-14-15-9(17)18-8)13-11-5-6-2-1-3-10-4-6/h1-5H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221270

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)C(=NNC(=O)Nc3nnc(S)s3)c2c1 |w:11.11| Show InChI InChI=1S/C11H7N7O4S2/c19-8-7(5-3-4(18(21)22)1-2-6(5)12-8)14-15-9(20)13-10-16-17-11(23)24-10/h1-3H,(H,17,23)(H,12,14,19)(H2,13,15,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221249
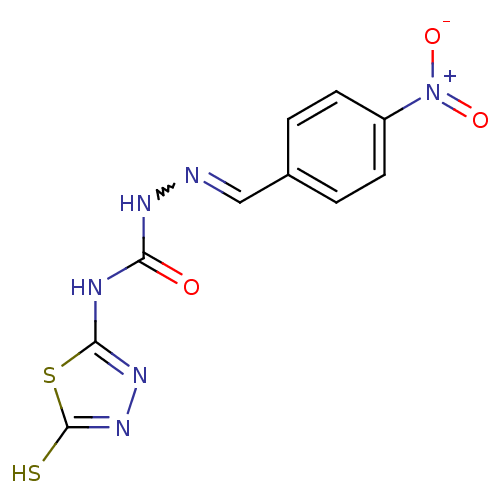
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES [O-][N+](=O)c1ccc(C=NNC(=O)Nc2nnc(S)s2)cc1 |w:8.8| Show InChI InChI=1S/C10H8N6O3S2/c17-8(12-9-14-15-10(20)21-9)13-11-5-6-1-3-7(4-2-6)16(18)19/h1-5H,(H,15,20)(H2,12,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221250
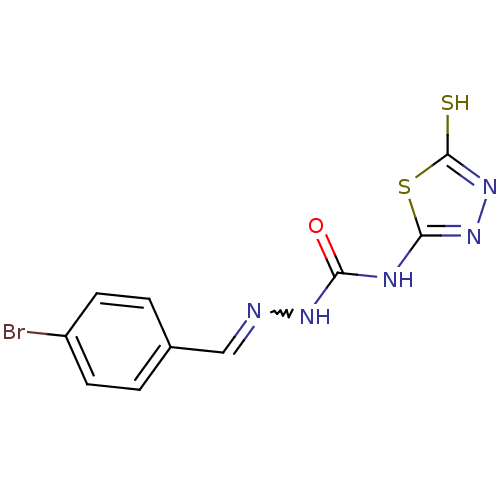
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C10H8BrN5OS2/c11-7-3-1-6(2-4-7)5-12-14-8(17)13-9-15-16-10(18)19-9/h1-5H,(H,16,18)(H2,13,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221252

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C11H11N5O2S2/c1-18-8-4-2-3-7(5-8)6-12-14-9(17)13-10-15-16-11(19)20-10/h2-6H,1H3,(H,16,19)(H2,13,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221269

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES [#16]-c1nnc(-[#7]-[#6](=O)-[#7]\[#7]=[#6](\c2ccccc2)-c2ccccc2)s1 Show InChI InChI=1S/C16H13N5OS2/c22-14(17-15-20-21-16(23)24-15)19-18-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,21,23)(H2,17,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221260
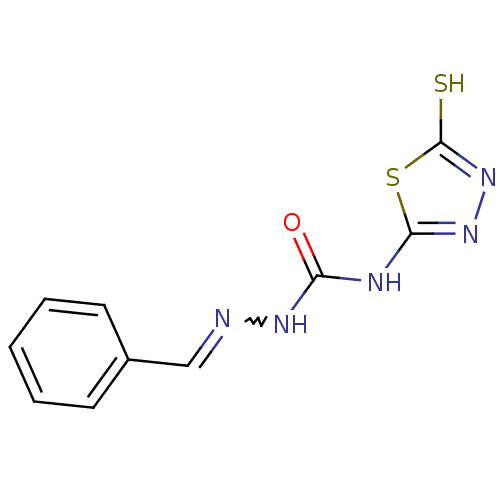
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show InChI InChI=1S/C10H9N5OS2/c16-8(12-9-14-15-10(17)18-9)13-11-6-7-4-2-1-3-5-7/h1-6H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221261

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C10H8ClN5OS2/c11-7-4-2-1-3-6(7)5-12-14-8(17)13-9-15-16-10(18)19-9/h1-5H,(H,16,18)(H2,13,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221266

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C11H11N5O2S2/c1-6(7-2-4-8(17)5-3-7)13-14-9(18)12-10-15-16-11(19)20-10/h2-5,17H,1H3,(H,16,19)(H2,12,14,15,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221255
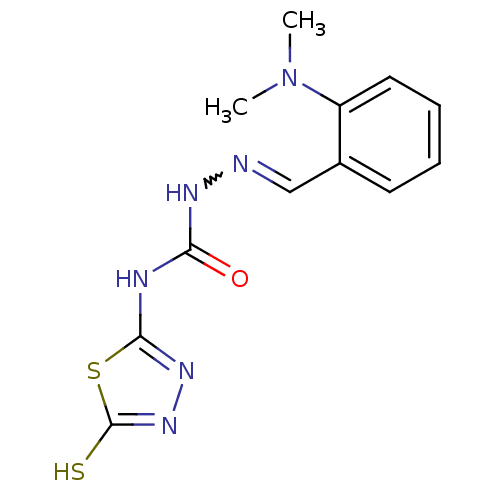
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES CN(C)c1ccccc1C=NNC(=O)Nc1nnc(S)s1 |w:10.11| Show InChI InChI=1S/C12H14N6OS2/c1-18(2)9-6-4-3-5-8(9)7-13-15-10(19)14-11-16-17-12(20)21-11/h3-7H,1-2H3,(H,17,20)(H2,14,15,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221264

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES CC(=NNC(=O)Nc1nnc(S)s1)c1ccc(cc1)[N+]([O-])=O |w:2.2| Show InChI InChI=1S/C11H10N6O3S2/c1-6(7-2-4-8(5-3-7)17(19)20)13-14-9(18)12-10-15-16-11(21)22-10/h2-5H,1H3,(H,16,21)(H2,12,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221263

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C12H13N5OS2/c1-7-3-5-9(6-4-7)8(2)14-15-10(18)13-11-16-17-12(19)20-11/h3-6H,1-2H3,(H,17,19)(H2,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221267

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C11H11N5OS2/c1-7(8-5-3-2-4-6-8)13-14-9(17)12-10-15-16-11(18)19-10/h2-6H,1H3,(H,16,18)(H2,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221253
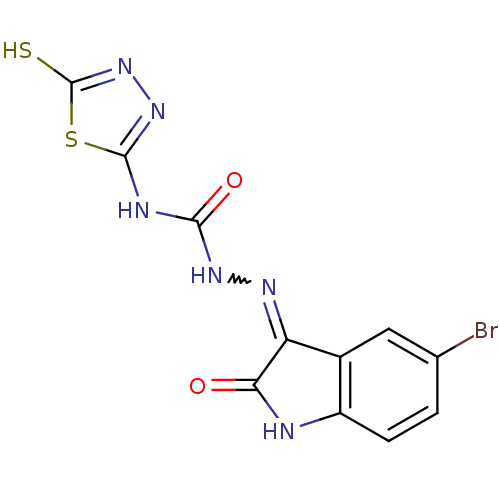
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show SMILES Sc1nnc(NC(=O)NN=C2C(=O)Nc3ccc(Br)cc23)s1 |w:9.8| Show InChI InChI=1S/C11H7BrN6O2S2/c12-4-1-2-6-5(3-4)7(8(19)13-6)15-16-9(20)14-10-17-18-11(21)22-10/h1-3H,(H,18,21)(H,13,15,19)(H2,14,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221271
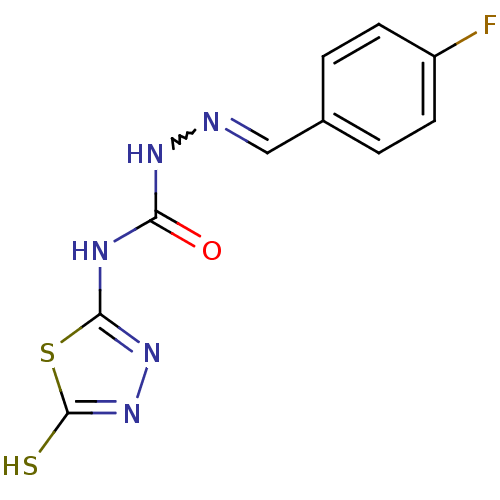
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C10H8FN5OS2/c11-7-3-1-6(2-4-7)5-12-14-8(17)13-9-15-16-10(18)19-9/h1-5H,(H,16,18)(H2,13,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221256
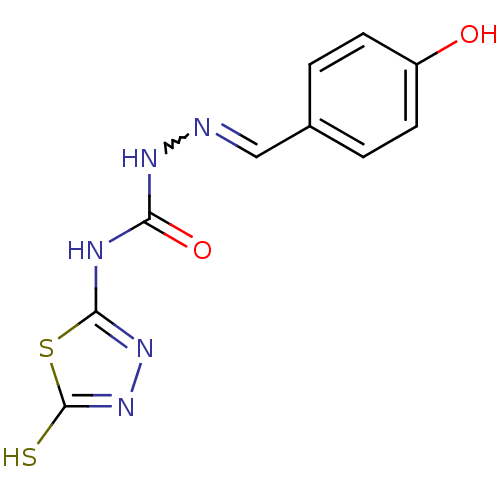
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C10H9N5O2S2/c16-7-3-1-6(2-4-7)5-11-13-8(17)12-9-14-15-10(18)19-9/h1-5,16H,(H,15,18)(H2,12,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50221265

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES CC(=NNC(=O)Nc1nnc(S)s1)c1ccc(Cl)cc1 |w:2.2| Show InChI InChI=1S/C11H10ClN5OS2/c1-6(7-2-4-8(12)5-3-7)14-15-9(18)13-10-16-17-11(19)20-10/h2-5H,1H3,(H,17,19)(H2,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase2 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221248

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES COc1ccc(cc1)C(C)=NNC(=O)Nc1nnc(S)s1 |w:10.11| Show InChI InChI=1S/C12H13N5O2S2/c1-7(8-3-5-9(19-2)6-4-8)14-15-10(18)13-11-16-17-12(20)21-11/h3-6H,1-2H3,(H,17,20)(H2,13,15,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221263

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C12H13N5OS2/c1-7-3-5-9(6-4-7)8(2)14-15-10(18)13-11-16-17-12(19)20-11/h3-6H,1-2H3,(H,17,19)(H2,13,15,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221269

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES [#16]-c1nnc(-[#7]-[#6](=O)-[#7]\[#7]=[#6](\c2ccccc2)-c2ccccc2)s1 Show InChI InChI=1S/C16H13N5OS2/c22-14(17-15-20-21-16(23)24-15)19-18-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,21,23)(H2,17,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221273

(4-(4,5-dyl)-1-[alpha-(3-pyridyl)methylene]semicarb...)Show InChI InChI=1S/C9H8N6OS2/c16-7(12-8-14-15-9(17)18-8)13-11-5-6-2-1-3-10-4-6/h1-5H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221254
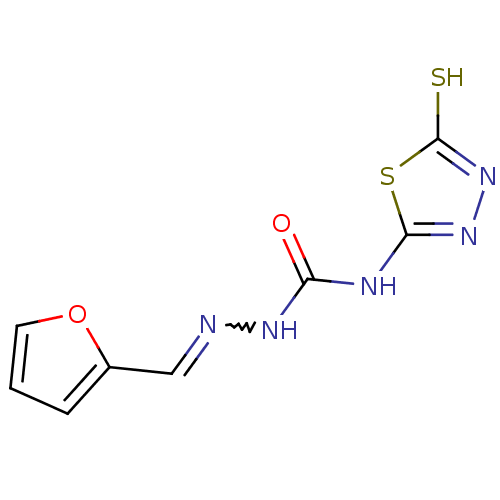
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C8H7N5O2S2/c14-6(10-7-12-13-8(16)17-7)11-9-4-5-2-1-3-15-5/h1-4H,(H,13,16)(H2,10,11,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221270

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)C(=NNC(=O)Nc3nnc(S)s3)c2c1 |w:11.11| Show InChI InChI=1S/C11H7N7O4S2/c19-8-7(5-3-4(18(21)22)1-2-6(5)12-8)14-15-9(20)13-10-16-17-11(23)24-10/h1-3H,(H,17,23)(H,12,14,19)(H2,13,15,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221272
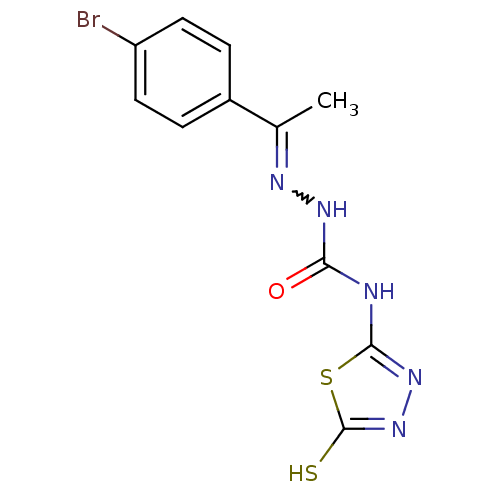
(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES CC(=NNC(=O)Nc1nnc(S)s1)c1ccc(Br)cc1 |w:2.2| Show InChI InChI=1S/C11H10BrN5OS2/c1-6(7-2-4-8(12)5-3-7)14-15-9(18)13-10-16-17-11(19)20-10/h2-5H,1H3,(H,17,19)(H2,13,15,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221274
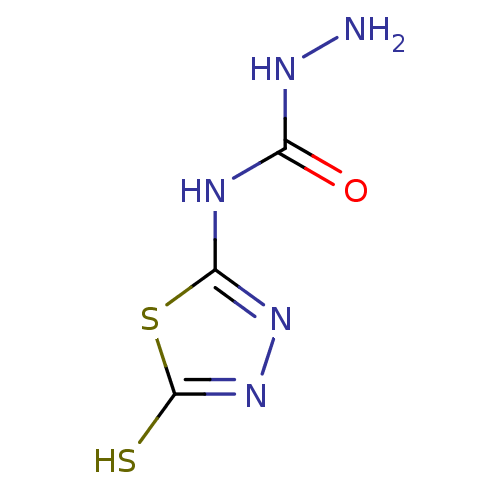
(CHEMBL237422 | ethyl N-(5-thioxo-4,5-dihydro-1,3,4...)Show InChI InChI=1S/C3H5N5OS2/c4-6-1(9)5-2-7-8-3(10)11-2/h4H2,(H,8,10)(H2,5,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50221249

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show SMILES [O-][N+](=O)c1ccc(C=NNC(=O)Nc2nnc(S)s2)cc1 |w:8.8| Show InChI InChI=1S/C10H8N6O3S2/c17-8(12-9-14-15-10(20)21-9)13-11-5-6-1-3-7(4-2-6)16(18)19/h1-5H,(H,15,20)(H2,12,13,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase1 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221252

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C11H11N5O2S2/c1-18-8-4-2-3-7(5-8)6-12-14-9(17)13-10-15-16-11(19)20-10/h2-6H,1H3,(H,16,19)(H2,13,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221256

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-[...)Show InChI InChI=1S/C10H9N5O2S2/c16-7-3-1-6(2-4-7)5-11-13-8(17)12-9-14-15-10(18)19-9/h1-5,16H,(H,15,18)(H2,12,13,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50221253

(4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...)Show SMILES Sc1nnc(NC(=O)NN=C2C(=O)Nc3ccc(Br)cc23)s1 |w:9.8| Show InChI InChI=1S/C11H7BrN6O2S2/c12-4-1-2-6-5(3-4)7(8(19)13-6)15-16-9(20)14-10-17-18-11(21)22-10/h1-3H,(H,18,21)(H,13,15,19)(H2,14,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Assiut University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration method |
Bioorg Med Chem 15: 6975-84 (2007)
Article DOI: 10.1016/j.bmc.2007.07.044
BindingDB Entry DOI: 10.7270/Q2P26XV5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data