 Found 148 hits with Last Name = 'abdel-meguid' and Initial = 'ss'
Found 148 hits with Last Name = 'abdel-meguid' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519
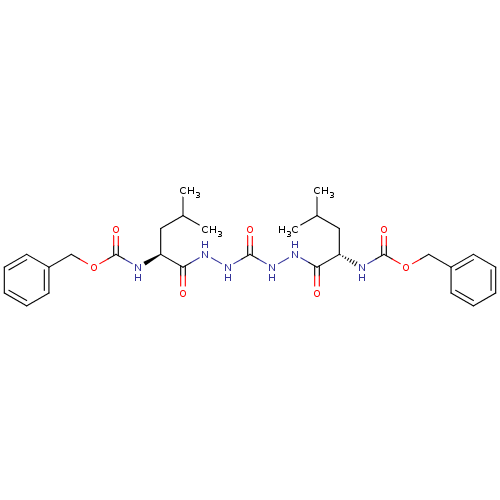
(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408522
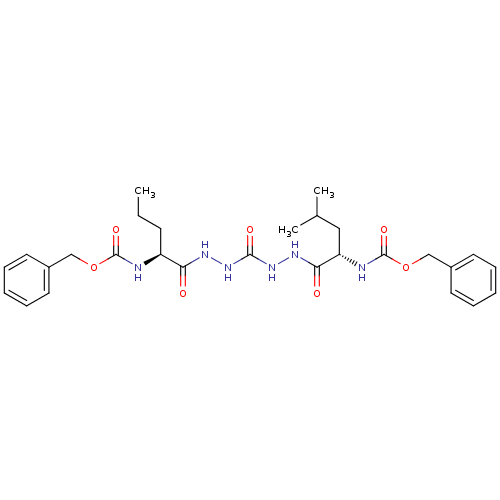
(CHEMBL126820)Show SMILES CCC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C28H38N6O7/c1-4-11-22(29-27(38)40-17-20-12-7-5-8-13-20)24(35)31-33-26(37)34-32-25(36)23(16-19(2)3)30-28(39)41-18-21-14-9-6-10-15-21/h5-10,12-15,19,22-23H,4,11,16-18H2,1-3H3,(H,29,38)(H,30,39)(H,31,35)(H,32,36)(H2,33,34,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408520
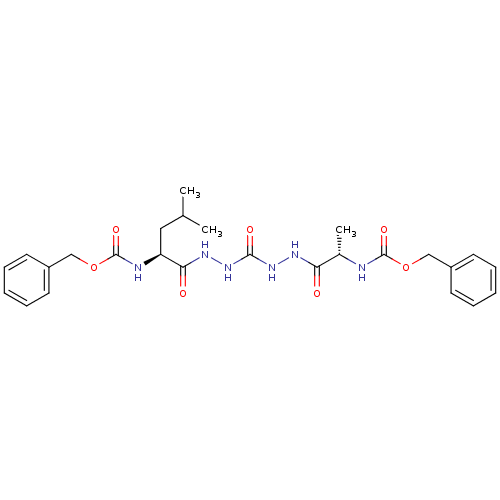
(CHEMBL126352)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C26H34N6O7/c1-17(2)14-21(28-26(37)39-16-20-12-8-5-9-13-20)23(34)30-32-24(35)31-29-22(33)18(3)27-25(36)38-15-19-10-6-4-7-11-19/h4-13,17-18,21H,14-16H2,1-3H3,(H,27,36)(H,28,37)(H,29,33)(H,30,34)(H2,31,32,35)/t18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
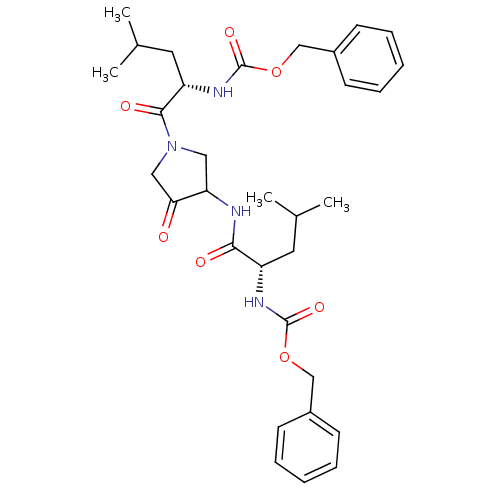
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19810
(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408521
(CHEMBL129773)Show SMILES CC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H36N6O7/c1-4-21(28-26(37)39-16-19-11-7-5-8-12-19)23(34)30-32-25(36)33-31-24(35)22(15-18(2)3)29-27(38)40-17-20-13-9-6-10-14-20/h5-14,18,21-22H,4,15-17H2,1-3H3,(H,28,37)(H,29,38)(H,30,34)(H,31,35)(H2,32,33,36)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066648
(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408515
(CHEMBL338770)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C29H33N5O6/c1-20(2)17-24(30-29(38)40-19-22-13-7-4-8-14-22)27(36)32-34-28(37)33-31-26(35)23-15-9-10-16-25(23)39-18-21-11-5-3-6-12-21/h3-16,20,24H,17-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650
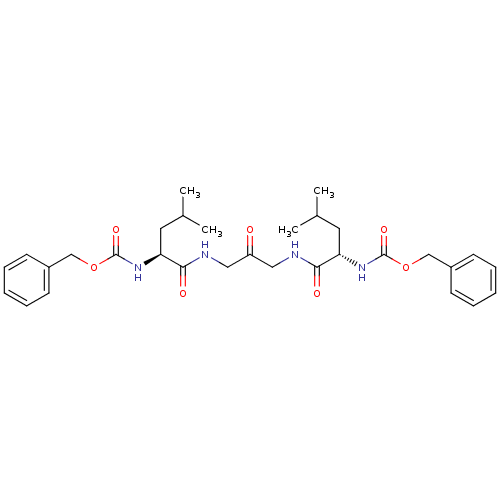
(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against cathepsin K. |
J Med Chem 41: 4567-76 (1998)
Article DOI: 10.1021/jm980249f
BindingDB Entry DOI: 10.7270/Q26974TN |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650

(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066645
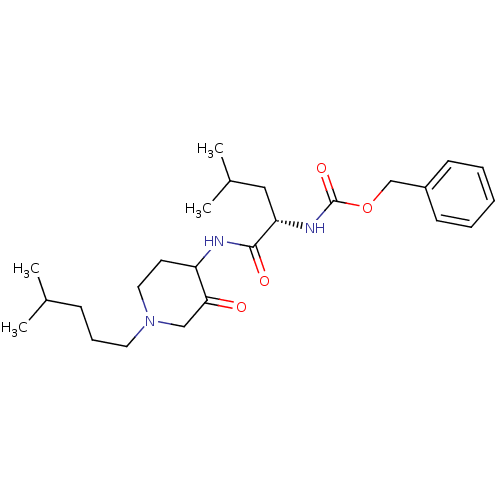
(CHEMBL432634 | {(S)-3-Methyl-1-[1-(4-methyl-pentyl...)Show SMILES CC(C)CCCN1CCC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C(=O)C1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)9-8-13-28-14-12-21(23(29)16-28)26-24(30)22(15-19(3)4)27-25(31)32-17-20-10-6-5-7-11-20/h5-7,10-11,18-19,21-22H,8-9,12-17H2,1-4H3,(H,26,30)(H,27,31)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408517
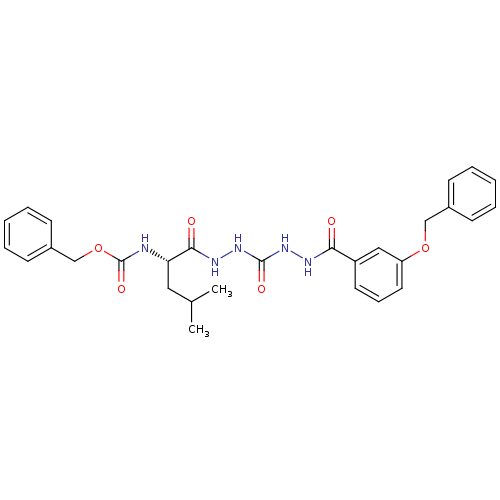
(CHEMBL340191)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1cccc(OCc2ccccc2)c1 |r| Show InChI InChI=1S/C29H33N5O6/c1-20(2)16-25(30-29(38)40-19-22-12-7-4-8-13-22)27(36)32-34-28(37)33-31-26(35)23-14-9-15-24(17-23)39-18-21-10-5-3-6-11-21/h3-15,17,20,25H,16,18-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066653
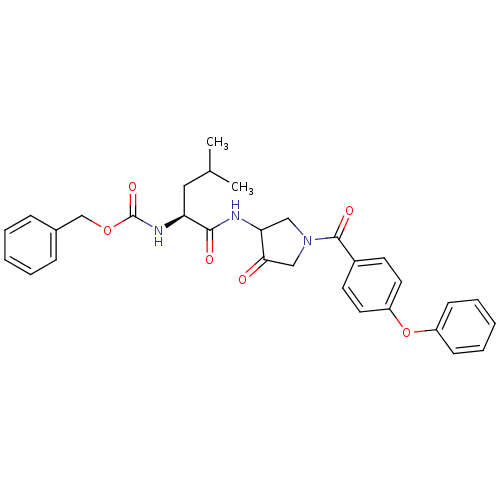
(CHEMBL119219 | {(S)-3-Methyl-1-[4-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)29(36)32-27-18-34(19-28(27)35)30(37)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19807

(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066653

(CHEMBL119219 | {(S)-3-Methyl-1-[4-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)29(36)32-27-18-34(19-28(27)35)30(37)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066654
(CHEMBL404801 | {(S)-1-[(1S,3S)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H]1CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C33H44N4O7/c1-21(2)17-27(36-32(41)43-19-23-11-7-5-8-12-23)30(39)34-25-15-16-26(29(25)38)35-31(40)28(18-22(3)4)37-33(42)44-20-24-13-9-6-10-14-24/h5-14,21-22,25-28H,15-20H2,1-4H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408518
(CHEMBL338150)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H33N5O6/c1-20(2)17-25(30-29(38)40-19-22-11-7-4-8-12-22)27(36)32-34-28(37)33-31-26(35)23-13-15-24(16-14-23)39-18-21-9-5-3-6-10-21/h3-16,20,25H,17-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066648

(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408516
(CHEMBL126835)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1ccccc1 Show InChI InChI=1S/C22H27N5O5/c1-15(2)13-18(23-22(31)32-14-16-9-5-3-6-10-16)20(29)25-27-21(30)26-24-19(28)17-11-7-4-8-12-17/h3-12,15,18H,13-14H2,1-2H3,(H,23,31)(H,24,28)(H,25,29)(H2,26,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066652

(CHEMBL117054 | {(S)-3-Methyl-1-[3-oxo-4-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N1CC(NC(=O)c2ccc(Oc3ccccc3)cc2)C(=O)C1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)30(37)34-18-27(28(35)19-34)32-29(36)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066652

(CHEMBL117054 | {(S)-3-Methyl-1-[3-oxo-4-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N1CC(NC(=O)c2ccc(Oc3ccccc3)cc2)C(=O)C1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)30(37)34-18-27(28(35)19-34)32-29(36)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066651

(CHEMBL118347 | {(S)-1-[(3R,4R)-4-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H46N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-29,38H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26-,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19807

(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066648

(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066646

(CHEMBL267064 | {(S)-1-[(3R,4R)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H44N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-28,37H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50066645

(CHEMBL432634 | {(S)-3-Methyl-1-[1-(4-methyl-pentyl...)Show SMILES CC(C)CCCN1CCC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C(=O)C1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)9-8-13-28-14-12-21(23(29)16-28)26-24(30)22(15-19(3)4)27-25(31)32-17-20-10-6-5-7-11-20/h5-7,10-11,18-19,21-22H,8-9,12-17H2,1-4H3,(H,26,30)(H,27,31)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066646

(CHEMBL267064 | {(S)-1-[(3R,4R)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H44N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-28,37H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066645

(CHEMBL432634 | {(S)-3-Methyl-1-[1-(4-methyl-pentyl...)Show SMILES CC(C)CCCN1CCC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C(=O)C1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)9-8-13-28-14-12-21(23(29)16-28)26-24(30)22(15-19(3)4)27-25(31)32-17-20-10-6-5-7-11-20/h5-7,10-11,18-19,21-22H,8-9,12-17H2,1-4H3,(H,26,30)(H,27,31)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066651

(CHEMBL118347 | {(S)-1-[(3R,4R)-4-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H46N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-29,38H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26-,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066653

(CHEMBL119219 | {(S)-3-Methyl-1-[4-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)29(36)32-27-18-34(19-28(27)35)30(37)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50066652

(CHEMBL117054 | {(S)-3-Methyl-1-[3-oxo-4-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N1CC(NC(=O)c2ccc(Oc3ccccc3)cc2)C(=O)C1 Show InChI InChI=1S/C31H33N3O6/c1-21(2)17-26(33-31(38)39-20-22-9-5-3-6-10-22)30(37)34-18-27(28(35)19-34)32-29(36)23-13-15-25(16-14-23)40-24-11-7-4-8-12-24/h3-16,21,26-27H,17-20H2,1-2H3,(H,32,36)(H,33,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066646

(CHEMBL267064 | {(S)-1-[(3R,4R)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H44N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-28,37H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066651

(CHEMBL118347 | {(S)-1-[(3R,4R)-4-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCN(C[C@H]1O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H46N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-29,38H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26-,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50066650

(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against papain. |
J Med Chem 41: 4567-76 (1998)
Article DOI: 10.1021/jm980249f
BindingDB Entry DOI: 10.7270/Q26974TN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066649
(CHEMBL117790 | {(S)-1-[(1R,3R)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C34H46N4O7/c1-22(2)18-28(37-33(42)44-20-24-12-7-5-8-13-24)31(40)35-26-16-11-17-27(30(26)39)36-32(41)29(19-23(3)4)38-34(43)45-21-25-14-9-6-10-15-25/h5-10,12-15,22-23,26-29H,11,16-21H2,1-4H3,(H,35,40)(H,36,41)(H,37,42)(H,38,43)/t26-,27-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066655

(CHEMBL325379 | {(S)-1-[(1S,3S)-3-((S)-2-Benzyloxyc...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H]1CCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C34H46N4O7/c1-22(2)18-28(37-33(42)44-20-24-12-7-5-8-13-24)31(40)35-26-16-11-17-27(30(26)39)36-32(41)29(19-23(3)4)38-34(43)45-21-25-14-9-6-10-15-25/h5-10,12-15,22-23,26-29H,11,16-21H2,1-4H3,(H,35,40)(H,36,41)(H,37,42)(H,38,43)/t26-,27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12972

((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C32H41BrN8O5S/c1-18(2)26(29(45)39-24(5-4-14-37-31(34)35)27(43)30-36-15-16-47-30)41-28(44)25(17-20-6-12-23(42)13-7-20)40-32(46)38-19(3)21-8-10-22(33)11-9-21/h6-13,15-16,18-19,24-26,42H,4-5,14,17H2,1-3H3,(H,39,45)(H,41,44)(H4,34,35,37)(H2,38,40,46)/t19-,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12973

((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C29H44BrN11O4S/c1-16(2)22(25(44)39-20(6-4-12-36-27(31)32)23(42)26-35-14-15-46-26)41-24(43)21(7-5-13-37-28(33)34)40-29(45)38-17(3)18-8-10-19(30)11-9-18/h8-11,14-17,20-22H,4-7,12-13H2,1-3H3,(H,39,44)(H,41,43)(H4,31,32,36)(H4,33,34,37)(H2,38,40,45)/t17-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12974
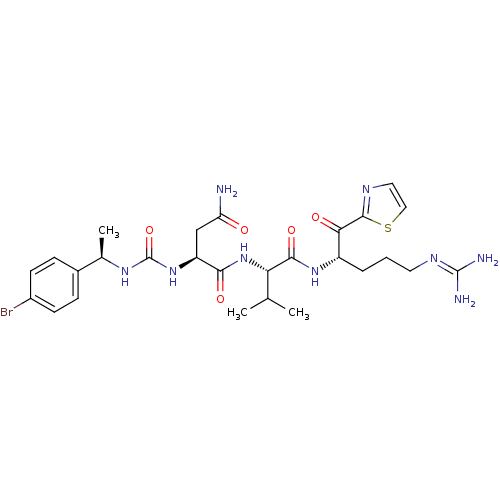
((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C27H38BrN9O5S/c1-14(2)21(24(41)35-18(5-4-10-33-26(30)31)22(39)25-32-11-12-43-25)37-23(40)19(13-20(29)38)36-27(42)34-15(3)16-6-8-17(28)9-7-16/h6-9,11-12,14-15,18-19,21H,4-5,10,13H2,1-3H3,(H2,29,38)(H,35,41)(H,37,40)(H4,30,31,33)(H2,34,36,42)/t15-,18+,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12962
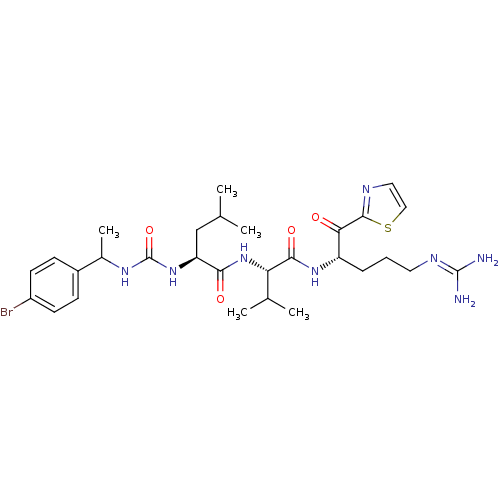
((2S)-2-({[1-(4-bromophenyl)ethyl]carbamoyl}amino)-...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#7]-[#6](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C29H43BrN8O4S/c1-16(2)15-22(37-29(42)35-18(5)19-8-10-20(30)11-9-19)25(40)38-23(17(3)4)26(41)36-21(7-6-12-34-28(31)32)24(39)27-33-13-14-43-27/h8-11,13-14,16-18,21-23H,6-7,12,15H2,1-5H3,(H,36,41)(H,38,40)(H4,31,32,34)(H2,35,37,42)/t18?,21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12968

((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C31H45BrN8O4S/c1-18(2)24(27(42)38-23(10-7-15-36-30(33)34)26(41)29-35-16-17-45-29)39-28(43)25(21-8-5-4-6-9-21)40-31(44)37-19(3)20-11-13-22(32)14-12-20/h11-14,16-19,21,23-25H,4-10,15H2,1-3H3,(H,38,42)(H,39,43)(H4,33,34,36)(H2,37,40,44)/t19-,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12977
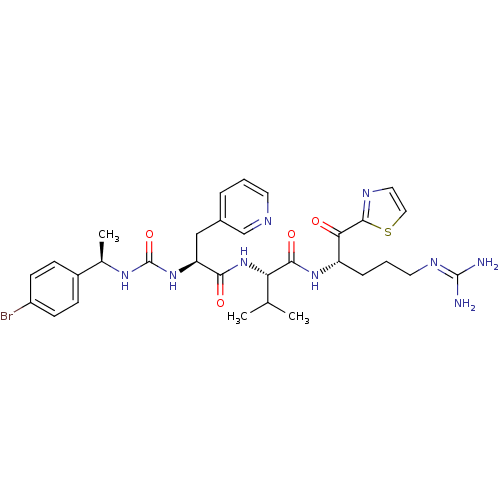
((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C31H40BrN9O4S/c1-18(2)25(28(44)39-23(7-5-13-37-30(33)34)26(42)29-36-14-15-46-29)41-27(43)24(16-20-6-4-12-35-17-20)40-31(45)38-19(3)21-8-10-22(32)11-9-21/h4,6,8-12,14-15,17-19,23-25H,5,7,13,16H2,1-3H3,(H,39,44)(H,41,43)(H4,33,34,37)(H2,38,40,45)/t19-,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12970
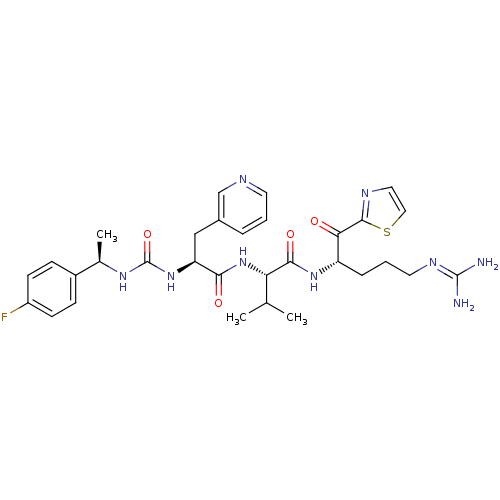
((2S)-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thiazo...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(F)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C31H40FN9O4S/c1-18(2)25(28(44)39-23(7-5-13-37-30(33)34)26(42)29-36-14-15-46-29)41-27(43)24(16-20-6-4-12-35-17-20)40-31(45)38-19(3)21-8-10-22(32)11-9-21/h4,6,8-12,14-15,17-19,23-25H,5,7,13,16H2,1-3H3,(H,39,44)(H,41,43)(H4,33,34,37)(H2,38,40,45)/t19-,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12975
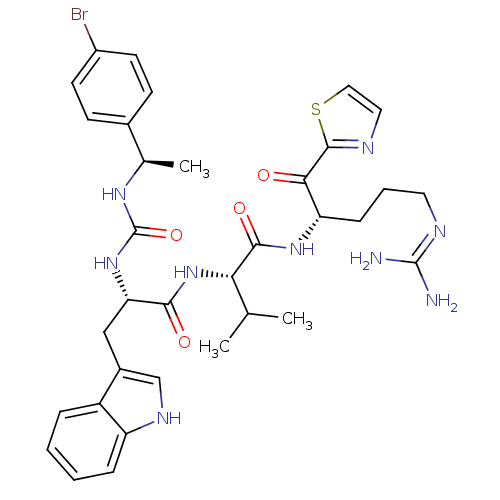
((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)N[C@H](C)c1ccc(Br)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)c1nccs1 |r,wU:7.19,34.36,22.24,wD:3.3,(-2.47,-.39,;-1.14,.38,;.19,-.39,;-1.14,1.92,;-2.47,2.69,;-3.81,1.92,;-3.81,.38,;-5.14,2.69,;-5.14,4.23,;-5.54,5.72,;-4.62,6.96,;-5.52,8.21,;-6.99,7.75,;-8.31,8.53,;-9.65,7.77,;-9.67,6.23,;-8.34,5.45,;-7,6.21,;-6.47,1.92,;-7.81,2.69,;-7.81,4.23,;-9.14,1.92,;-10.48,2.69,;-10.48,4.23,;-11.81,1.92,;-13.14,2.69,;-14.48,1.92,;-14.48,.38,;-15.81,-.39,;-13.14,-.39,;-11.81,.38,;.19,2.69,;.19,4.23,;1.53,1.92,;2.86,2.69,;2.86,4.23,;4.19,5,;4.19,6.54,;2.86,7.31,;2.86,8.85,;4.19,9.62,;1.53,9.62,;4.19,1.92,;4.19,.38,;5.53,2.69,;7.07,2.69,;7.54,4.16,;6.3,5.06,;5.05,4.16,)| Show InChI InChI=1S/C34H42BrN9O4S/c1-19(2)28(31(47)42-26(9-6-14-39-33(36)37)29(45)32-38-15-16-49-32)44-30(46)27(17-22-18-40-25-8-5-4-7-24(22)25)43-34(48)41-20(3)21-10-12-23(35)13-11-21/h4-5,7-8,10-13,15-16,18-20,26-28,40H,6,9,14,17H2,1-3H3,(H,42,47)(H,44,46)(H4,36,37,39)(H2,41,43,48)/t20-,26+,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12976
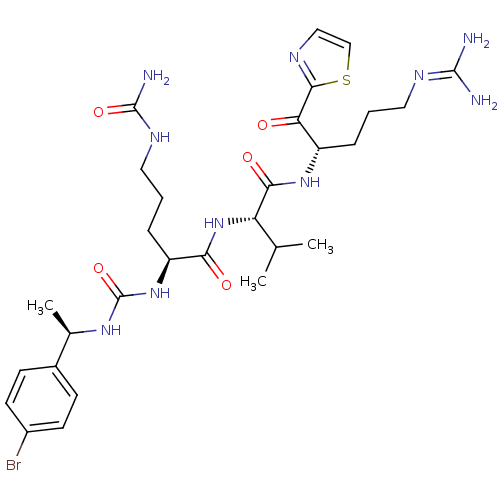
((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C29H43BrN10O5S/c1-16(2)22(25(43)38-20(6-4-12-35-27(31)32)23(41)26-34-14-15-46-26)40-24(42)21(7-5-13-36-28(33)44)39-29(45)37-17(3)18-8-10-19(30)11-9-18/h8-11,14-17,20-22H,4-7,12-13H2,1-3H3,(H,38,43)(H,40,42)(H4,31,32,35)(H3,33,36,44)(H2,37,39,45)/t17-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data