 Found 156 hits with Last Name = 'abdollahi' and Initial = 'm'
Found 156 hits with Last Name = 'abdollahi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50406864

(CHEMBL4161244)Show SMILES COc1ccc(cc1OC)-c1cc2ccc(OCCCn3cc(CNC(=O)CCCCC4CCSS4)nn3)cc2oc1=O Show InChI InChI=1S/C31H36N4O6S2/c1-38-27-11-9-21(17-29(27)39-2)26-16-22-8-10-24(18-28(22)41-31(26)37)40-14-5-13-35-20-23(33-34-35)19-32-30(36)7-4-3-6-25-12-15-42-43-25/h8-11,16-18,20,25H,3-7,12-15,19H2,1-2H3,(H,32,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate pretreated for 5 mins followed by subst... |
Eur J Med Chem 152: 600-614 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.058
BindingDB Entry DOI: 10.7270/Q2WW7M8B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50155425
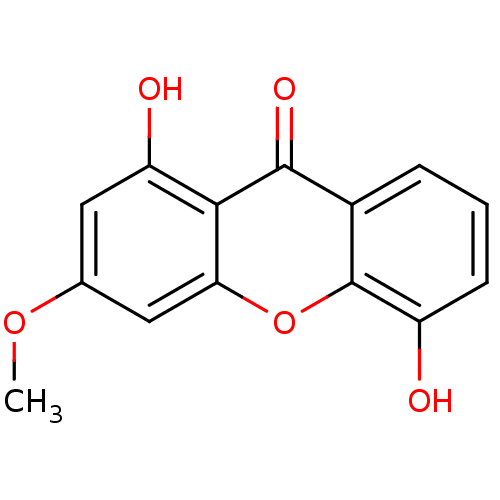
(1,5-Dihydroxy-3-methoxy-xanthen-9-one | CHEMBL3637...)Show InChI InChI=1S/C14H10O5/c1-18-7-5-10(16)12-11(6-7)19-14-8(13(12)17)3-2-4-9(14)15/h2-6,15-16H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5282182

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50606816

(CHEMBL5219771)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2cccc(c2)-c2cc(=O)c3ccccc3o2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5278007

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5273737

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325191

((Z)-1-(2-Fluorobenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2F)cc1 Show InChI InChI=1S/C22H17FNO3/c1-26-17-6-7-18-20(13-17)27-21(22(18)25)12-15-8-10-24(11-9-15)14-16-4-2-3-5-19(16)23/h2-13H,14H2,1H3/q+1/b21-12- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5276955
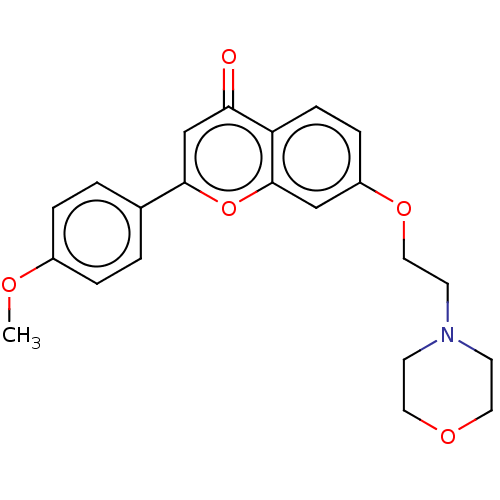
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's method |
Eur J Med Chem 152: 600-614 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.058
BindingDB Entry DOI: 10.7270/Q2WW7M8B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325193

((Z)-1-(4-Fluorobenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C22H17FNO3/c1-26-18-6-7-19-20(13-18)27-21(22(19)25)12-15-8-10-24(11-9-15)14-16-2-4-17(23)5-3-16/h2-13H,14H2,1H3/q+1/b21-12- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5273737

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5278007

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5284318
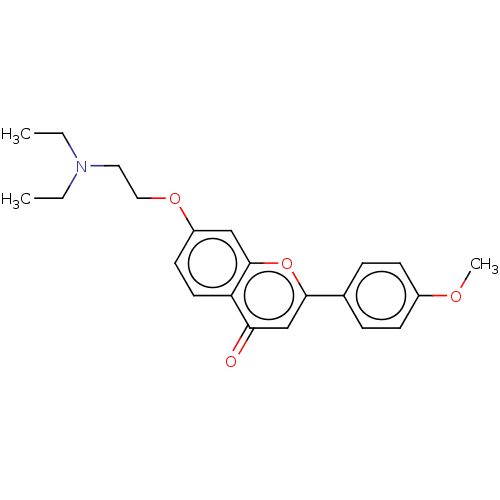
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325198

((Z)-1-(2-Fluorobenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2F)cc1 Show InChI InChI=1S/C23H19FNO3/c1-2-27-18-7-8-19-21(14-18)28-22(23(19)26)13-16-9-11-25(12-10-16)15-17-5-3-4-6-20(17)24/h3-14H,2,15H2,1H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50155425

(1,5-Dihydroxy-3-methoxy-xanthen-9-one | CHEMBL3637...)Show InChI InChI=1S/C14H10O5/c1-18-7-5-10(16)12-11(6-7)19-14-8(13(12)17)3-2-4-9(14)15/h2-6,15-16H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325190

((Z)-1-Benzyl-4-((6-methoxy-3-oxobenzofuran-2(3H)-y...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C22H18NO3/c1-25-18-7-8-19-20(14-18)26-21(22(19)24)13-16-9-11-23(12-10-16)15-17-5-3-2-4-6-17/h2-14H,15H2,1H3/q+1/b21-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325199
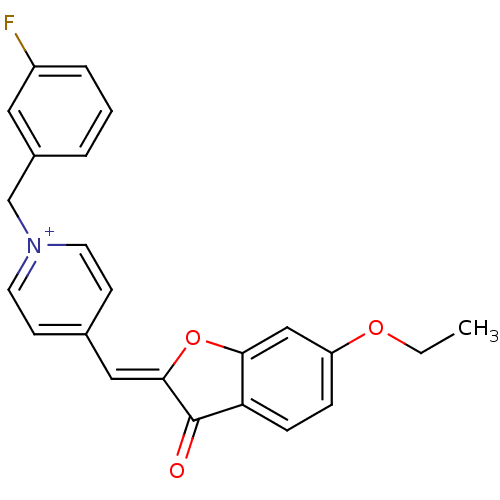
((Z)-1-(3-Fluorobenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(F)c2)cc1 Show InChI InChI=1S/C23H19FNO3/c1-2-27-19-6-7-20-21(14-19)28-22(23(20)26)13-16-8-10-25(11-9-16)15-17-4-3-5-18(24)12-17/h3-14H,2,15H2,1H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5284318

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325205

((Z)-1-(2-Fluorobenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2F)cc1 Show InChI InChI=1S/C24H21FNO3/c1-2-13-28-19-7-8-20-22(15-19)29-23(24(20)27)14-17-9-11-26(12-10-17)16-18-5-3-4-6-21(18)25/h3-12,14-15H,2,13,16H2,1H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325200

((Z)-1-(4-Fluorobenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H19FNO3/c1-2-27-19-7-8-20-21(14-19)28-22(23(20)26)13-16-9-11-25(12-10-16)15-17-3-5-18(24)6-4-17/h3-14H,2,15H2,1H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5276955

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325192

((Z)-1-(3-Fluorobenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(F)c2)cc1 Show InChI InChI=1S/C22H17FNO3/c1-26-18-5-6-19-20(13-18)27-21(22(19)25)12-15-7-9-24(10-8-15)14-16-3-2-4-17(23)11-16/h2-13H,14H2,1H3/q+1/b21-12- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325197

((Z)-1-Benzyl-4-((6-ethoxy-3-oxobenzofuran-2(3H)-yl...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C23H20NO3/c1-2-26-19-8-9-20-21(15-19)27-22(23(20)25)14-17-10-12-24(13-11-17)16-18-6-4-3-5-7-18/h3-15H,2,16H2,1H3/q+1/b22-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50606816

(CHEMBL5219771)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2cccc(c2)-c2cc(=O)c3ccccc3o2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325194

((Z)-1-(2-Methylbenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2C)cc1 Show InChI InChI=1S/C23H20NO3/c1-16-5-3-4-6-18(16)15-24-11-9-17(10-12-24)13-22-23(25)20-8-7-19(26-2)14-21(20)27-22/h3-14H,15H2,1-2H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325207
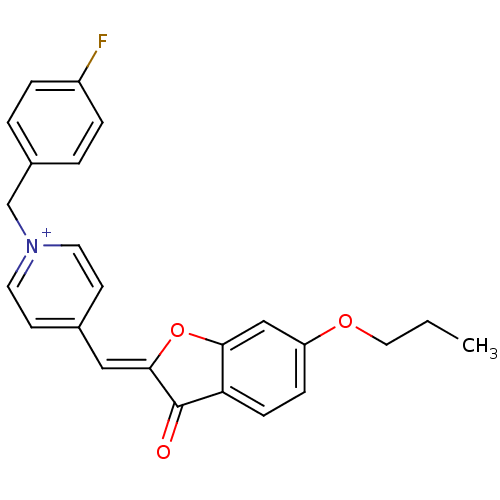
((Z)-1-(4-Fluorobenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C24H21FNO3/c1-2-13-28-20-7-8-21-22(15-20)29-23(24(21)27)14-17-9-11-26(12-10-17)16-18-3-5-19(25)6-4-18/h3-12,14-15H,2,13,16H2,1H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5285784
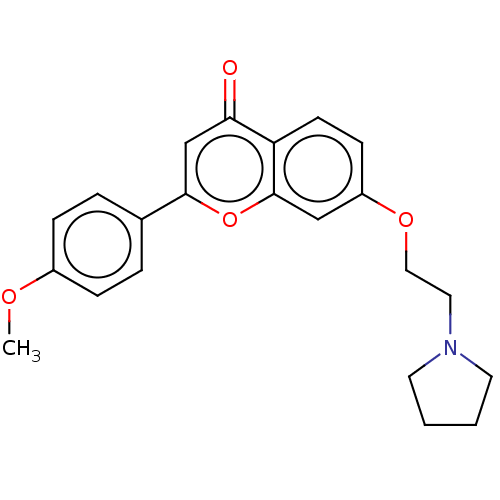
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325204

((Z)-1-Benzyl-4-((6-propoxy-3-oxobenzofuran-2(3H)-y...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C24H22NO3/c1-2-14-27-20-8-9-21-22(16-20)28-23(24(21)26)15-18-10-12-25(13-11-18)17-19-6-4-3-5-7-19/h3-13,15-16H,2,14,17H2,1H3/q+1/b23-15- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5286597

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5282182

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5285784

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325206

((Z)-1-(3-Fluorobenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(F)c2)cc1 Show InChI InChI=1S/C24H21FNO3/c1-2-12-28-20-6-7-21-22(15-20)29-23(24(21)27)14-17-8-10-26(11-9-17)16-18-4-3-5-19(25)13-18/h3-11,13-15H,2,12,16H2,1H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325195

((Z)-1-(3-Methylbenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(C)c2)cc1 Show InChI InChI=1S/C23H20NO3/c1-16-4-3-5-18(12-16)15-24-10-8-17(9-11-24)13-22-23(25)20-7-6-19(26-2)14-21(20)27-22/h3-14H,15H2,1-2H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | CHEMBL5290033
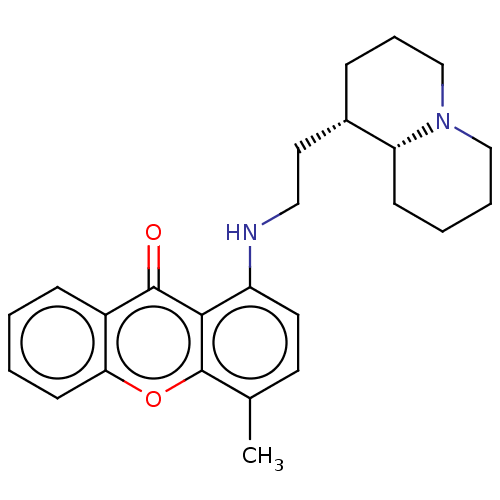
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325209

((Z)-1-(3-Methylbenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(C)c2)cc1 Show InChI InChI=1S/C25H24NO3/c1-3-13-28-21-7-8-22-23(16-21)29-24(25(22)27)15-19-9-11-26(12-10-19)17-20-6-4-5-18(2)14-20/h4-12,14-16H,3,13,17H2,1-2H3/q+1/b24-15- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325208
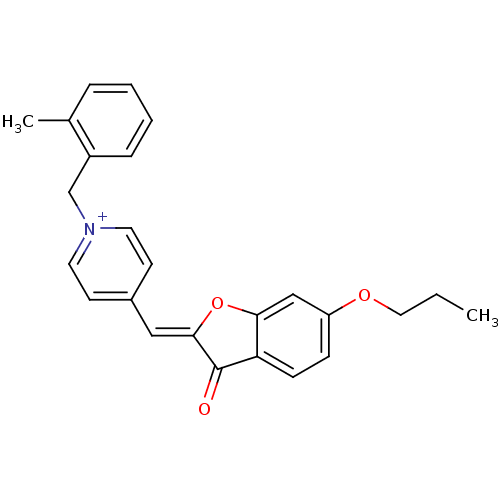
((Z)-1-(2-Methylbenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2C)cc1 Show InChI InChI=1S/C25H24NO3/c1-3-14-28-21-8-9-22-23(16-21)29-24(25(22)27)15-19-10-12-26(13-11-19)17-20-7-5-4-6-18(20)2/h4-13,15-16H,3,14,17H2,1-2H3/q+1/b24-15- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5290033

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325202

((Z)-1-(3-Methylbenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2cccc(C)c2)cc1 Show InChI InChI=1S/C24H22NO3/c1-3-27-20-7-8-21-22(15-20)28-23(24(21)26)14-18-9-11-25(12-10-18)16-19-6-4-5-17(2)13-19/h4-15H,3,16H2,1-2H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | CHEMBL5286183

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325196

((Z)-1-(4-Methylbenzyl)-4-((6-methoxy-3-oxobenzofur...)Show SMILES COc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(C)cc2)cc1 Show InChI InChI=1S/C23H20NO3/c1-16-3-5-18(6-4-16)15-24-11-9-17(10-12-24)13-22-23(25)20-8-7-19(26-2)14-21(20)27-22/h3-14H,15H2,1-2H3/q+1/b22-13- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 475 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325201

((Z)-1-(2-Methylbenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccccc2C)cc1 Show InChI InChI=1S/C24H22NO3/c1-3-27-20-8-9-21-22(15-20)28-23(24(21)26)14-18-10-12-25(13-11-18)16-19-7-5-4-6-17(19)2/h4-15H,3,16H2,1-2H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325210

((Z)-1-(4-Methylbenzyl)-4-((6-propoxy-3-oxobenzofur...)Show SMILES CCCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(C)cc2)cc1 Show InChI InChI=1S/C25H24NO3/c1-3-14-28-21-8-9-22-23(16-21)29-24(25(22)27)15-19-10-12-26(13-11-19)17-20-6-4-18(2)5-7-20/h4-13,15-16H,3,14,17H2,1-2H3/q+1/b24-15- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 612 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | CHEMBL5289680

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50325203
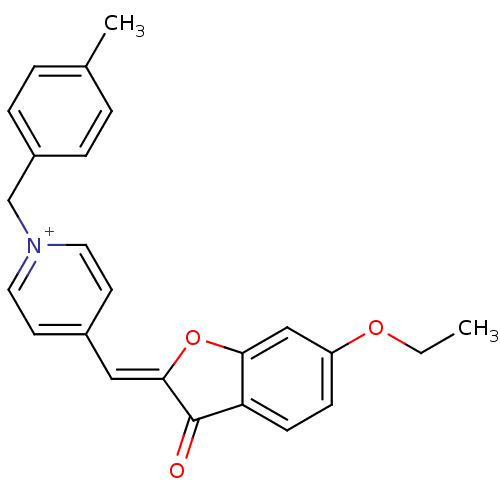
((Z)-1-(4-Methylbenzyl)-4-((6-ethoxy-3-oxobenzofura...)Show SMILES CCOc1ccc2C(=O)\C(Oc2c1)=C\c1cc[n+](Cc2ccc(C)cc2)cc1 Show InChI InChI=1S/C24H22NO3/c1-3-27-20-8-9-21-22(15-20)28-23(24(21)26)14-18-10-12-25(13-11-18)16-19-6-4-17(2)5-7-19/h4-15H,3,16H2,1-2H3/q+1/b23-14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 18: 6360-6 (2010)
Article DOI: 10.1016/j.bmc.2010.07.012
BindingDB Entry DOI: 10.7270/Q22807TN |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | CHEMBL5286183

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM167904
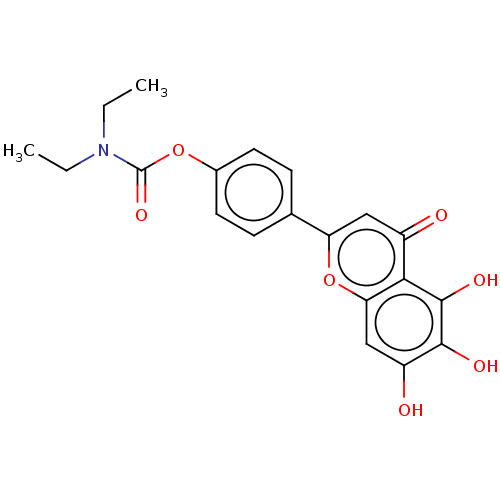
(N,N-diethylcarbamic acid-4-(5,6,7-trihydroxy-4-oxo...)Show SMILES CCN(CC)C(=O)Oc1ccc(cc1)-c1cc(=O)c2c(O)c(O)c(O)cc2o1 Show InChI InChI=1S/C20H19NO7/c1-3-21(4-2)20(26)27-12-7-5-11(6-8-12)15-9-13(22)17-16(28-15)10-14(23)18(24)19(17)25/h5-10,23-25H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data