 Found 136 hits with Last Name = 'abdulla' and Initial = 'mm'
Found 136 hits with Last Name = 'abdulla' and Initial = 'mm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072313

(CHEMBL3408393)Show SMILES O=C1N(\C(S\C1=C1/SC(=NN1c1ccccc1)c1ccccc1)=N\c1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C41H26N6OS2/c42-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)43-37(34)44-41-46(31-22-12-4-13-23-31)39(48)36(49-41)40-47(32-24-14-5-15-25-32)45-38(50-40)30-20-10-3-11-21-30/h1-26H/b40-36-,44-41- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202577
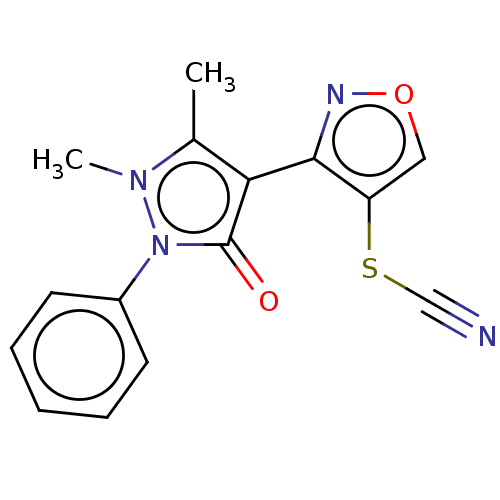
(3-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3-oxo-3H-p...)Show SMILES Cc1c(-c2nocc2SC#N)c(=O)n(-c2ccccc2)n1C |(16.74,-22.83,;15.33,-22.2,;15.01,-20.7,;16.04,-19.55,;17.57,-19.71,;18.2,-18.31,;17.06,-17.28,;15.72,-18.05,;14.32,-17.42,;14.15,-15.89,;14.15,-14.35,;13.48,-20.54,;12.71,-19.2,;12.85,-21.94,;11.35,-22.26,;10.32,-21.12,;8.81,-21.44,;8.33,-22.9,;9.37,-24.05,;10.87,-23.73,;14,-22.97,;13.84,-24.5,)| Show InChI InChI=1S/C15H12N4O2S/c1-10-13(14-12(22-9-16)8-21-17-14)15(20)19(18(10)2)11-6-4-3-5-7-11/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50089076

(CHEMBL3577642)Show SMILES COc1ccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3ccc(OC)cc3)c3ccccc3)cc2)cc1 |c:17| Show InChI InChI=1S/C32H27N3O5/c1-38-26-16-10-24(11-17-26)33-30(36)21-40-28-14-8-22(9-15-28)20-29-32(37)35(25-12-18-27(39-2)19-13-25)31(34-29)23-6-4-3-5-7-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072316
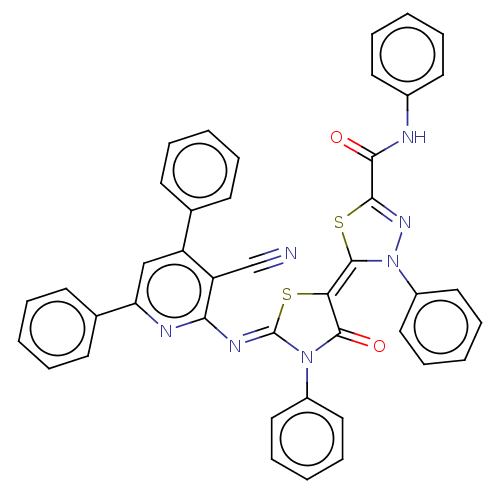
(CHEMBL3408396)Show SMILES O=C(Nc1ccccc1)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:10| Show InChI InChI=1S/C42H27N7O2S2/c43-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)45-37(34)46-42-48(31-22-12-4-13-23-31)40(51)36(52-42)41-49(32-24-14-5-15-25-32)47-39(53-41)38(50)44-30-20-10-3-11-21-30/h1-26H,(H,44,50)/b41-36-,46-42- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088858
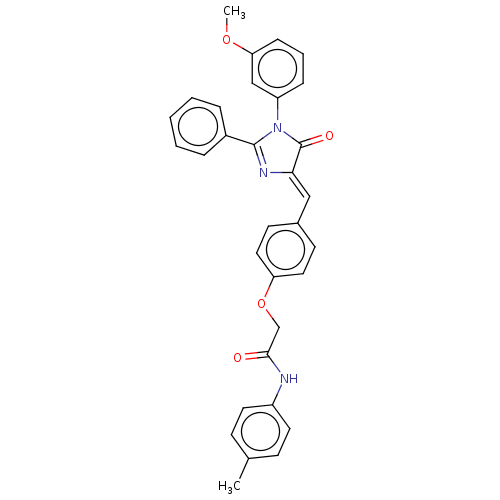
(CHEMBL3577566)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccc(C)cc3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C32H27N3O4/c1-22-11-15-25(16-12-22)33-30(36)21-39-27-17-13-23(14-18-27)19-29-32(37)35(26-9-6-10-28(20-26)38-2)31(34-29)24-7-4-3-5-8-24/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202580
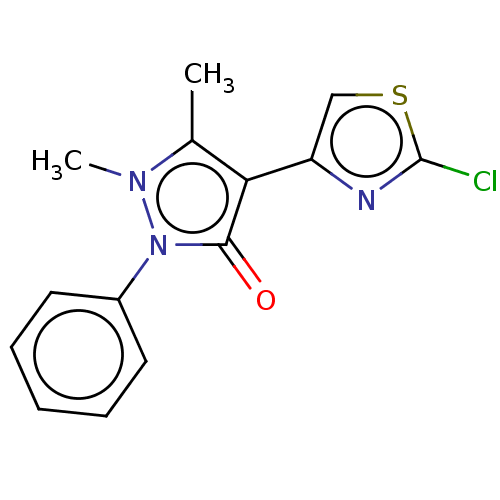
(2-Chloro-4-[4-(1,2-dihydro-1,5-dimethyl-2-phenyl-3...)Show InChI InChI=1S/C14H12ClN3OS/c1-9-12(11-8-20-14(15)16-11)13(19)18(17(9)2)10-6-4-3-5-7-10/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202578

(3-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyra...)Show SMILES CC1C(C(=O)N(N1C)c1ccccc1)c1csc2nc3ccccc3c(=O)n12 Show InChI InChI=1S/C21H18N4O2S/c1-13-18(20(27)25(23(13)2)14-8-4-3-5-9-14)17-12-28-21-22-16-11-7-6-10-15(16)19(26)24(17)21/h3-13,18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088791

(CHEMBL3577634)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(Cl)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-27-12-6-11-25(19-27)35-30(22-7-3-2-4-8-22)34-28(31(35)37)17-21-13-15-26(16-14-21)39-20-29(36)33-24-10-5-9-23(32)18-24/h2-19H,20H2,1H3,(H,33,36)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202579
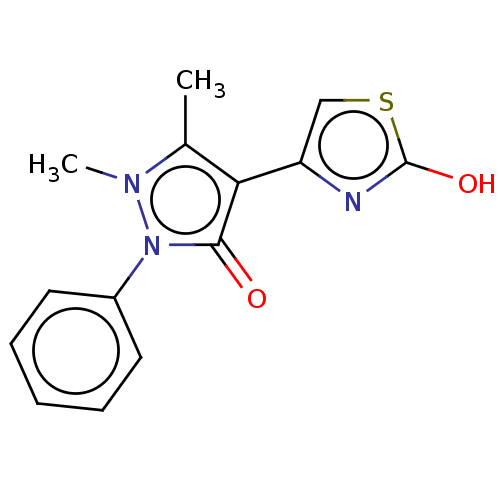
(2-Hydroxy-4-([4-(1,2-dihydro-1,5-dimethyl-2-phenyl...)Show InChI InChI=1S/C14H13N3O2S/c1-9-12(11-8-20-14(19)15-11)13(18)17(16(9)2)10-6-4-3-5-7-10/h3-8H,1-2H3,(H,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50089074
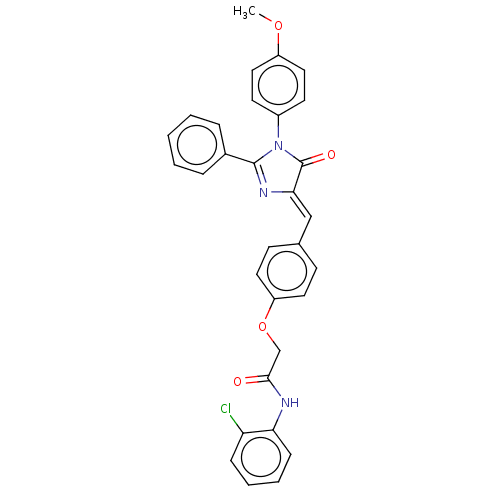
(CHEMBL3577643)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3Cl)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-24-17-13-23(14-18-24)35-30(22-7-3-2-4-8-22)34-28(31(35)37)19-21-11-15-25(16-12-21)39-20-29(36)33-27-10-6-5-9-26(27)32/h2-19H,20H2,1H3,(H,33,36)/b28-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072315
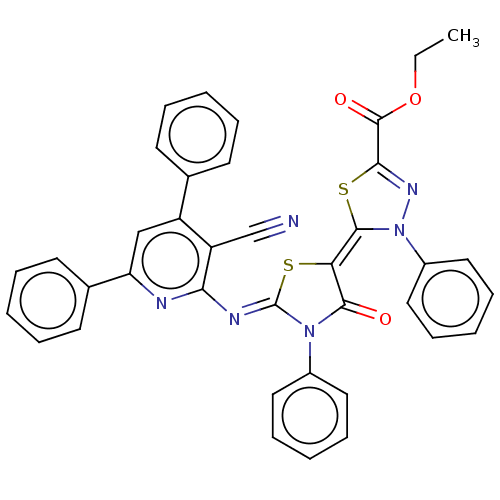
(CHEMBL3408395)Show SMILES CCOC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:5| Show InChI InChI=1S/C38H26N6O3S2/c1-2-47-37(46)34-42-44(28-21-13-6-14-22-28)36(49-34)32-35(45)43(27-19-11-5-12-20-27)38(48-32)41-33-30(24-39)29(25-15-7-3-8-16-25)23-31(40-33)26-17-9-4-10-18-26/h3-23H,2H2,1H3/b36-32-,41-38- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088791

(CHEMBL3577634)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(Cl)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-27-12-6-11-25(19-27)35-30(22-7-3-2-4-8-22)34-28(31(35)37)17-21-13-15-26(16-14-21)39-20-29(36)33-24-10-5-9-23(32)18-24/h2-19H,20H2,1H3,(H,33,36)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202576
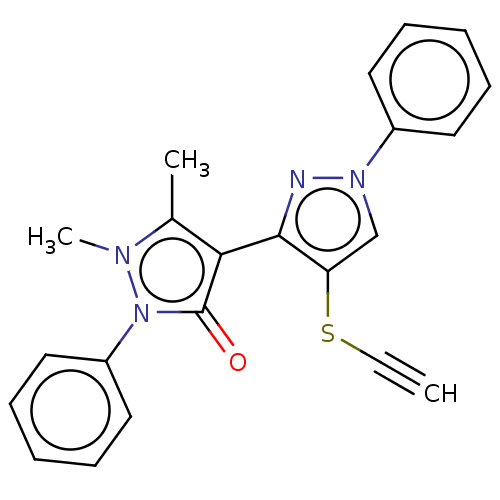
(1-Phenyl-3-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3...)Show SMILES Cc1c(-c2nn(cc2SC#C)-c2ccccc2)c(=O)n(-c2ccccc2)n1C |(6.84,-23.1,;5.43,-22.48,;5.11,-20.97,;6.14,-19.83,;7.39,-20.73,;8.63,-19.83,;8.16,-18.36,;6.62,-18.36,;5.71,-17.12,;6.34,-15.71,;6.74,-14.22,;10.1,-20.3,;10.42,-21.81,;11.88,-22.29,;13.03,-21.25,;12.71,-19.75,;11.24,-19.27,;3.58,-20.81,;2.81,-19.48,;2.95,-22.22,;1.45,-22.54,;.42,-21.39,;-1.09,-21.71,;-1.57,-23.18,;-.53,-24.32,;.97,-24,;4.1,-23.25,;3.94,-24.78,)| Show InChI InChI=1S/C22H18N4OS/c1-4-28-19-15-25(17-11-7-5-8-12-17)23-21(19)20-16(2)24(3)26(22(20)27)18-13-9-6-10-14-18/h1,5-15H,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202575
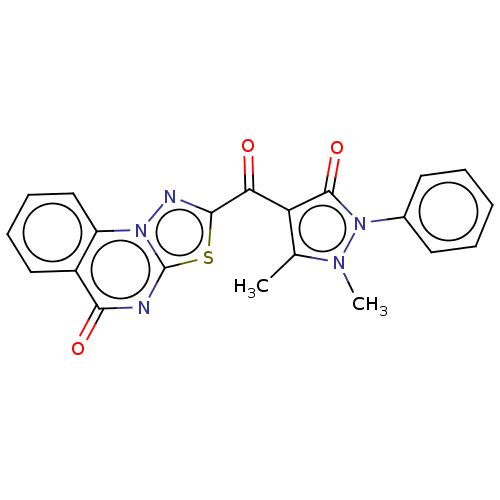
(2-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3-oxo-3H-p...)Show SMILES Cc1c(C(=O)c2nn3c(nc(=O)c4ccccc34)s2)c(=O)n(-c2ccccc2)n1C Show InChI InChI=1S/C21H15N5O3S/c1-12-16(20(29)26(24(12)2)13-8-4-3-5-9-13)17(27)19-23-25-15-11-7-6-10-14(15)18(28)22-21(25)30-19/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202574
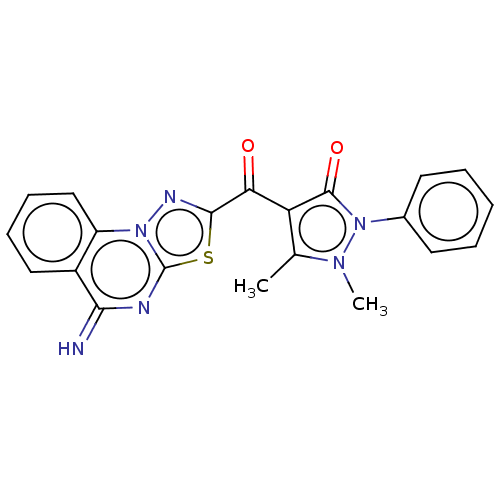
(2-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3-oxo-3H-p...)Show SMILES Cc1c(C(=O)c2nn3c(nc(=N)c4ccccc34)s2)c(=O)n(-c2ccccc2)n1C Show InChI InChI=1S/C21H16N6O2S/c1-12-16(20(29)27(25(12)2)13-8-4-3-5-9-13)17(28)19-24-26-15-11-7-6-10-14(15)18(22)23-21(26)30-19/h3-11,22H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088855

(CHEMBL3577632)Show SMILES COc1ccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3cccc(OC)c3)c3ccccc3)cc2)cc1 |c:17| Show InChI InChI=1S/C32H27N3O5/c1-38-26-17-13-24(14-18-26)33-30(36)21-40-27-15-11-22(12-16-27)19-29-32(37)35(25-9-6-10-28(20-25)39-2)31(34-29)23-7-4-3-5-8-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088855

(CHEMBL3577632)Show SMILES COc1ccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3cccc(OC)c3)c3ccccc3)cc2)cc1 |c:17| Show InChI InChI=1S/C32H27N3O5/c1-38-26-17-13-24(14-18-26)33-30(36)21-40-27-15-11-22(12-16-27)19-29-32(37)35(25-9-6-10-28(20-25)39-2)31(34-29)23-7-4-3-5-8-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088858

(CHEMBL3577566)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccc(C)cc3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C32H27N3O4/c1-22-11-15-25(16-12-22)33-30(36)21-39-27-17-13-23(14-18-27)19-29-32(37)35(26-9-6-10-28(20-26)38-2)31(34-29)24-7-4-3-5-8-24/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088897

(CHEMBL3577644)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(Cl)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-26-16-12-25(13-17-26)35-30(22-6-3-2-4-7-22)34-28(31(35)37)18-21-10-14-27(15-11-21)39-20-29(36)33-24-9-5-8-23(32)19-24/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088896

(CHEMBL3577645)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccc(Cl)cc3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-26-17-13-25(14-18-26)35-30(22-5-3-2-4-6-22)34-28(31(35)37)19-21-7-15-27(16-8-21)39-20-29(36)33-24-11-9-23(32)10-12-24/h2-19H,20H2,1H3,(H,33,36)/b28-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072314
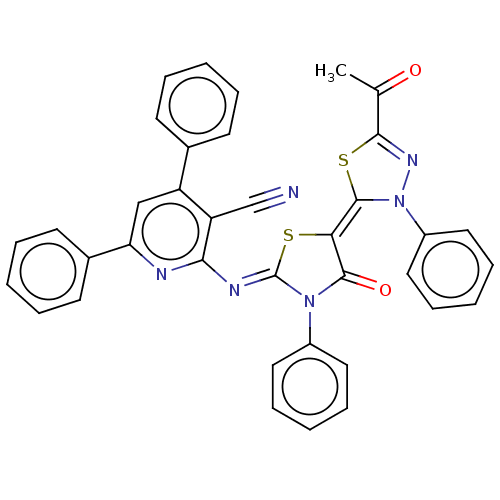
(CHEMBL3408394)Show SMILES CC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:3| Show InChI InChI=1S/C37H24N6O2S2/c1-24(44)34-41-43(28-20-12-5-13-21-28)36(47-34)32-35(45)42(27-18-10-4-11-19-27)37(46-32)40-33-30(23-38)29(25-14-6-2-7-15-25)22-31(39-33)26-16-8-3-9-17-26/h2-22H,1H3/b36-32-,40-37- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202573
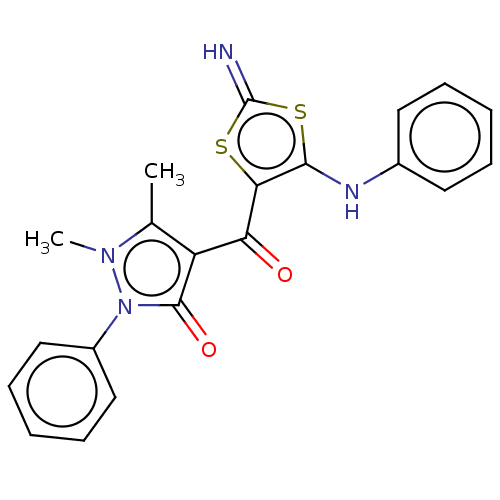
(4-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3-oxo-3H-p...)Show SMILES Cc1c(C(=O)c2sc(=N)sc2Nc2ccccc2)c(=O)n(-c2ccccc2)n1C Show InChI InChI=1S/C21H18N4O2S2/c1-13-16(20(27)25(24(13)2)15-11-7-4-8-12-15)17(26)18-19(29-21(22)28-18)23-14-9-5-3-6-10-14/h3-12,22-23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50089083

(CHEMBL3577635)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccc(Cl)cc3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-27-9-5-8-25(19-27)35-30(22-6-3-2-4-7-22)34-28(31(35)37)18-21-10-16-26(17-11-21)39-20-29(36)33-24-14-12-23(32)13-15-24/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50089083

(CHEMBL3577635)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccc(Cl)cc3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-27-9-5-8-25(19-27)35-30(22-6-3-2-4-7-22)34-28(31(35)37)18-21-10-16-26(17-11-21)39-20-29(36)33-24-14-12-23(32)13-15-24/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50089074

(CHEMBL3577643)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3Cl)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-24-17-13-23(14-18-24)35-30(22-7-3-2-4-8-22)34-28(31(35)37)19-21-11-15-25(16-12-21)39-20-29(36)33-27-10-6-5-9-26(27)32/h2-19H,20H2,1H3,(H,33,36)/b28-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088857
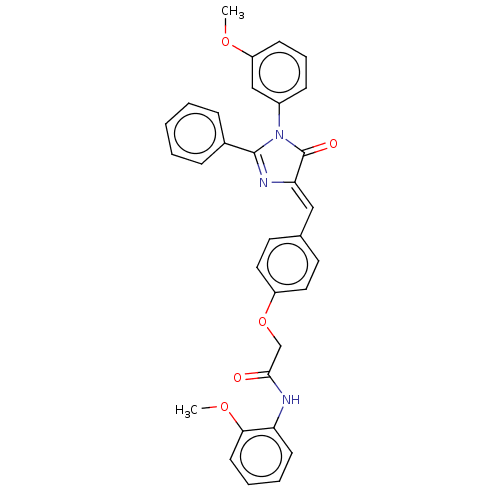
(CHEMBL3577567)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3OC)cc2)N=C1c1ccccc1 |c:35| Show InChI InChI=1S/C32H27N3O5/c1-38-26-12-8-11-24(20-26)35-31(23-9-4-3-5-10-23)34-28(32(35)37)19-22-15-17-25(18-16-22)40-21-30(36)33-27-13-6-7-14-29(27)39-2/h3-20H,21H2,1-2H3,(H,33,36)/b28-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088854
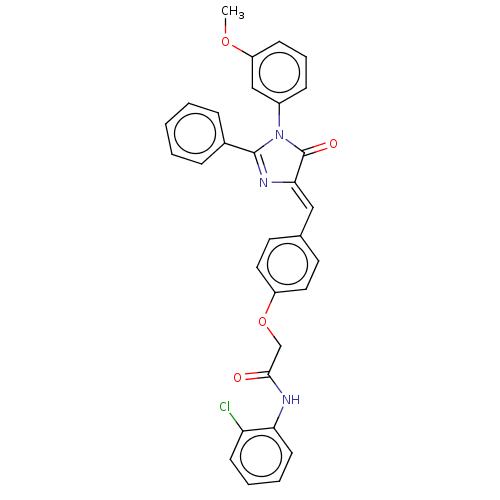
(CHEMBL3577633)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3Cl)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-25-11-7-10-23(19-25)35-30(22-8-3-2-4-9-22)34-28(31(35)37)18-21-14-16-24(17-15-21)39-20-29(36)33-27-13-6-5-12-26(27)32/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202572
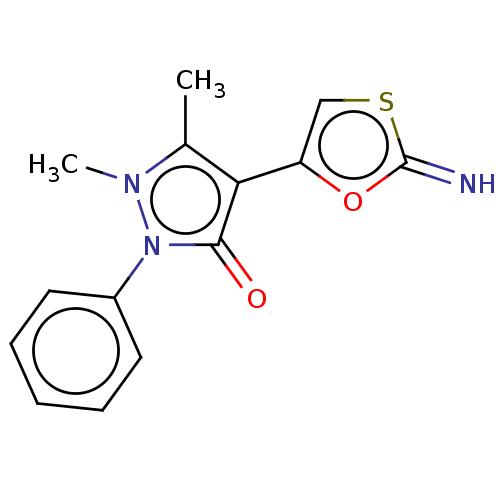
(6-[4-(1,2-Dihydro-1,5-dimethyl-2-phenyl-3-oxo-3H-p...)Show InChI InChI=1S/C14H13N3O2S/c1-9-12(11-8-20-14(15)19-11)13(18)17(16(9)2)10-6-4-3-5-7-10/h3-8,15H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088856

(CHEMBL3577631)Show SMILES COc1cccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3cccc(OC)c3)c3ccccc3)cc2)c1 |c:18| Show InChI InChI=1S/C32H27N3O5/c1-38-27-12-6-10-24(19-27)33-30(36)21-40-26-16-14-22(15-17-26)18-29-32(37)35(25-11-7-13-28(20-25)39-2)31(34-29)23-8-4-3-5-9-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088854

(CHEMBL3577633)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3Cl)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-25-11-7-10-23(19-25)35-30(22-8-3-2-4-9-22)34-28(31(35)37)18-21-14-16-24(17-15-21)39-20-29(36)33-27-13-6-5-12-26(27)32/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50088856

(CHEMBL3577631)Show SMILES COc1cccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3cccc(OC)c3)c3ccccc3)cc2)c1 |c:18| Show InChI InChI=1S/C32H27N3O5/c1-38-27-12-6-10-24(19-27)33-30(36)21-40-26-16-14-22(15-17-26)18-29-32(37)35(25-11-7-13-28(20-25)39-2)31(34-29)23-8-4-3-5-9-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM202571
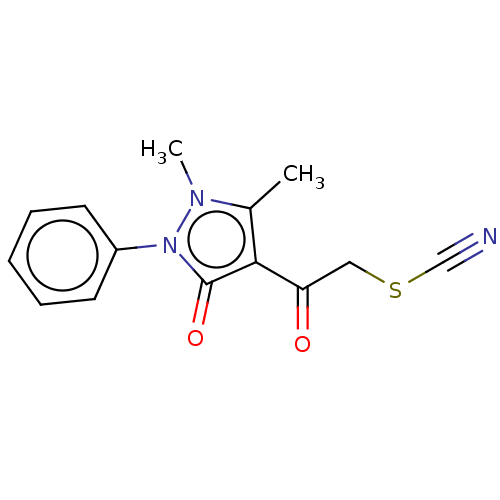
(1,2-Dihydro-1,5-dimethyl-2-phenyl-4-thiocyanatoace...)Show InChI InChI=1S/C14H13N3O2S/c1-10-13(12(18)8-20-9-15)14(19)17(16(10)2)11-6-4-3-5-7-11/h3-7H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yueyang Vocational Technical College
| Assay Description
This fluorescence-based assay measures the rate at which recombinant
human aromatase (baculovirus/insect cell-expressed) converts the
substrate 7-m... |
Chem Biol Drug Des 88: 832-843 (2016)
Article DOI: 10.1111/cbdd.12812
BindingDB Entry DOI: 10.7270/Q2HM578V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50088897

(CHEMBL3577644)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(Cl)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C31H24ClN3O4/c1-38-26-16-12-25(13-17-26)35-30(22-6-3-2-4-7-22)34-28(31(35)37)18-21-10-14-27(15-11-21)39-20-29(36)33-24-9-5-8-23(32)19-24/h2-19H,20H2,1H3,(H,33,36)/b28-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072312

(CHEMBL3408392)Show SMILES N#Cc1c(\N=c2/scc(-c3ccccc3)n2-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C33H22N4S/c34-22-29-28(24-13-5-1-6-14-24)21-30(25-15-7-2-8-16-25)35-32(29)36-33-37(27-19-11-4-12-20-27)31(23-38-33)26-17-9-3-10-18-26/h1-21,23H/b36-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17054
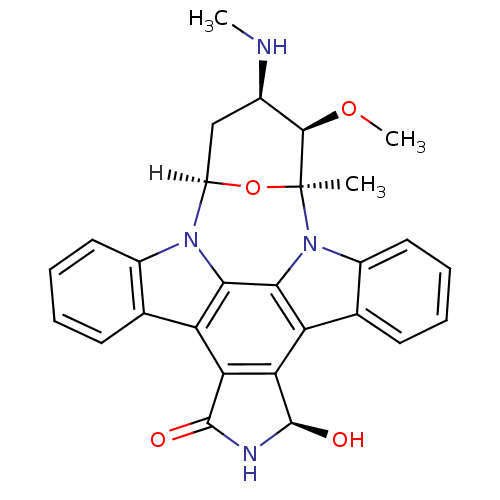
((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072281
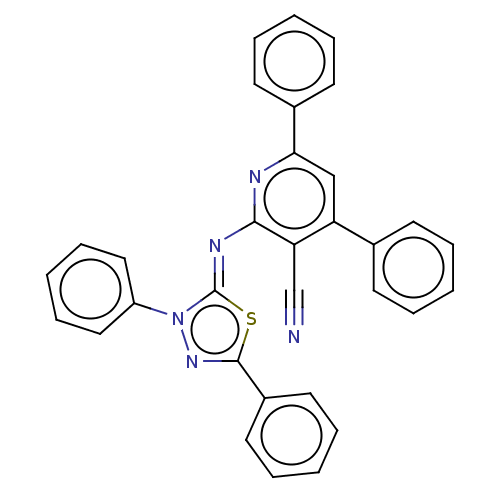
(CHEMBL3408382)Show SMILES N#Cc1c(\N=c2/sc(nn2-c2ccccc2)-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C32H21N5S/c33-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)34-30(28)35-32-37(26-19-11-4-12-20-26)36-31(38-32)25-17-9-3-10-18-25/h1-21H/b35-32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-ÁL reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072310
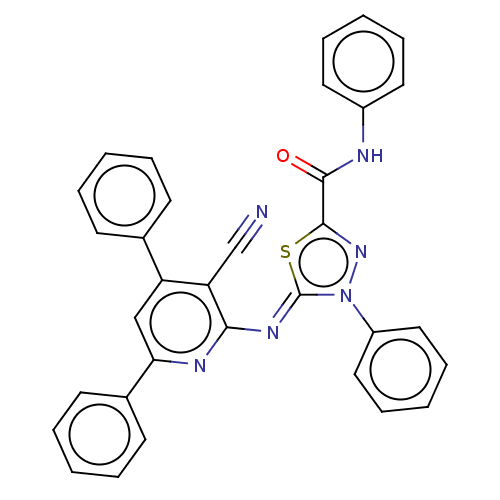
(CHEMBL3408390)Show SMILES O=C(Nc1ccccc1)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C33H22N6OS/c34-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)36-30(28)37-33-39(26-19-11-4-12-20-26)38-32(41-33)31(40)35-25-17-9-3-10-18-25/h1-21H,(H,35,40)/b37-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072309

(CHEMBL3408389)Show SMILES CCOC(=O)c1nn(-c2ccc(Cl)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H20ClN5O2S/c1-2-37-28(36)27-34-35(22-15-13-21(30)14-16-22)29(38-27)33-26-24(18-31)23(19-9-5-3-6-10-19)17-25(32-26)20-11-7-4-8-12-20/h3-17H,2H2,1H3/b33-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167935
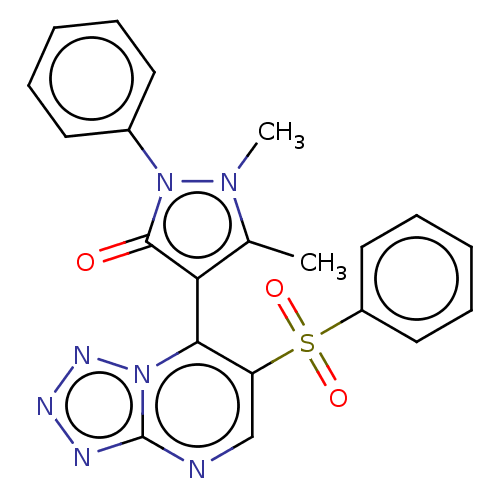
(1,5-Dimethyl-2-phenyl-4-(6-(phenylsulfonyl)tetrazo...)Show SMILES Cc1c(-c2c(cnc3nnnn23)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(2.21,6.35,;3.68,6.83,;4.92,5.92,;4.92,4.38,;6.26,3.61,;6.26,2.07,;4.92,1.3,;3.59,2.07,;2.13,1.6,;1.22,2.84,;2.13,4.09,;3.59,3.61,;7.59,4.38,;6.82,5.72,;8.36,3.05,;8.92,5.15,;8.92,6.69,;10.26,7.46,;11.59,6.69,;11.59,5.15,;10.26,4.38,;6.17,6.83,;7.63,6.35,;5.69,8.29,;6.6,9.54,;5.97,10.95,;6.88,12.19,;8.41,12.03,;9.04,10.62,;8.13,9.38,;4.15,8.29,;3.25,9.54,)| Show InChI InChI=1S/C21H17N7O3S/c1-14-18(20(29)28(26(14)2)15-9-5-3-6-10-15)19-17(13-22-21-23-24-25-27(19)21)32(30,31)16-11-7-4-8-12-16/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-ÁL reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072308

(CHEMBL3408388)Show SMILES CCOC(=O)c1nn(-c2ccc(C)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C30H23N5O2S/c1-3-37-29(36)28-34-35(23-16-14-20(2)15-17-23)30(38-28)33-27-25(19-31)24(21-10-6-4-7-11-21)18-26(32-27)22-12-8-5-9-13-22/h4-18H,3H2,1-2H3/b33-30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167938

(1',5'-Dimethyl-2'-phenyl-5-(phenylsulf...)Show SMILES Cc1c(-c2cc(n[nH]2)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C Show InChI InChI=1S/C20H18N4O3S/c1-14-19(20(25)24(23(14)2)15-9-5-3-6-10-15)17-13-18(22-21-17)28(26,27)16-11-7-4-8-12-16/h3-13H,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-ÁL reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072307
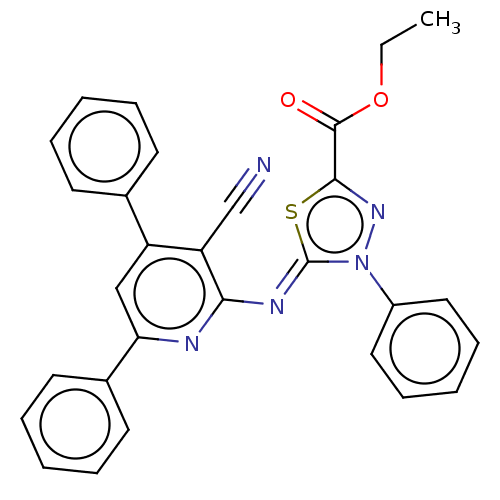
(CHEMBL3408387)Show SMILES CCOC(=O)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H21N5O2S/c1-2-36-28(35)27-33-34(22-16-10-5-11-17-22)29(37-27)32-26-24(19-30)23(20-12-6-3-7-13-20)18-25(31-26)21-14-8-4-9-15-21/h3-18H,2H2,1H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072306

(CHEMBL3408386)Show SMILES CC(=O)c1nn(-c2ccc(Cl)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C28H18ClN5OS/c1-18(35)27-33-34(22-14-12-21(29)13-15-22)28(36-27)32-26-24(17-30)23(19-8-4-2-5-9-19)16-25(31-26)20-10-6-3-7-11-20/h2-16H,1H3/b32-28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072305

(CHEMBL3408385)Show SMILES COc1ccc(cc1)-n1nc(s\c1=N/c1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1)C(C)=O Show InChI InChI=1S/C29H21N5O2S/c1-19(35)28-33-34(22-13-15-23(36-2)16-14-22)29(37-28)32-27-25(18-30)24(20-9-5-3-6-10-20)17-26(31-27)21-11-7-4-8-12-21/h3-17H,1-2H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072304
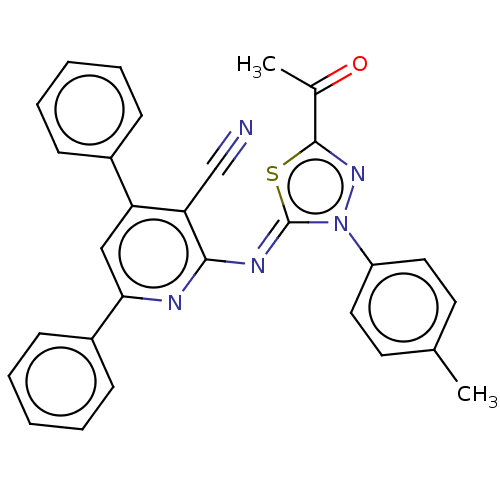
(CHEMBL3408384)Show SMILES CC(=O)c1nn(-c2ccc(C)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H21N5OS/c1-19-13-15-23(16-14-19)34-29(36-28(33-34)20(2)35)32-27-25(18-30)24(21-9-5-3-6-10-21)17-26(31-27)22-11-7-4-8-12-22/h3-17H,1-2H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50088859
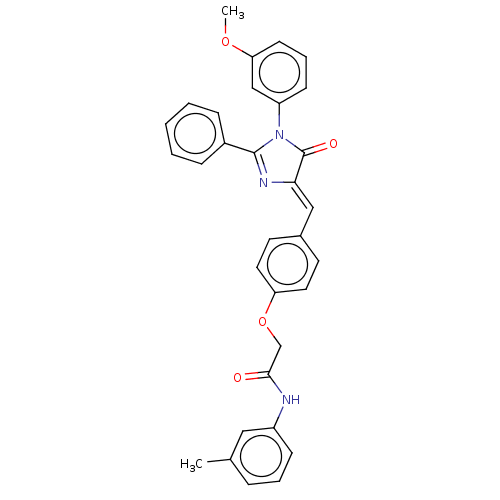
(CHEMBL3577565)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(C)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C32H27N3O4/c1-22-8-6-11-25(18-22)33-30(36)21-39-27-16-14-23(15-17-27)19-29-32(37)35(26-12-7-13-28(20-26)38-2)31(34-29)24-9-4-3-5-10-24/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50089077
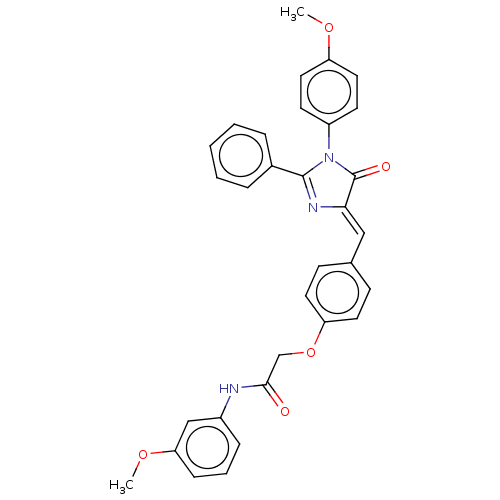
(CHEMBL3577641)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(OC)c3)cc2)N=C1c1ccccc1 |c:35| Show InChI InChI=1S/C32H27N3O5/c1-38-26-17-13-25(14-18-26)35-31(23-7-4-3-5-8-23)34-29(32(35)37)19-22-11-15-27(16-12-22)40-21-30(36)33-24-9-6-10-28(20-24)39-2/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data