

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM227453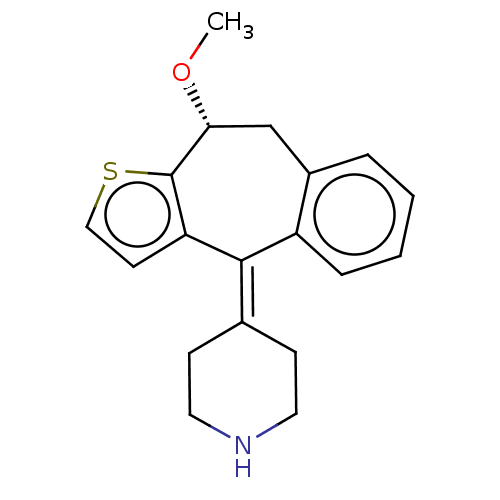 (US9333199, S-NORKETOTIFEN (SNORK)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM181150 (US9138431, R-NORKETOTIFEN (RNORK) | US9138431, RS-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM227454 (US9333199, R-NORKETOTIFEN (RNORK)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50017674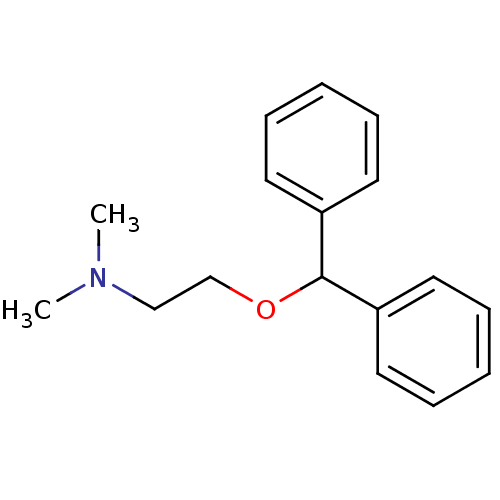 ((2-Benzhydryloxy-ethyl)-dimethyl-amine | 2-(benzhy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50073179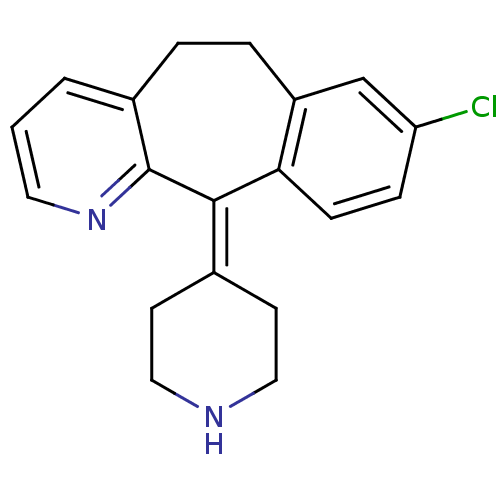 (8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM94597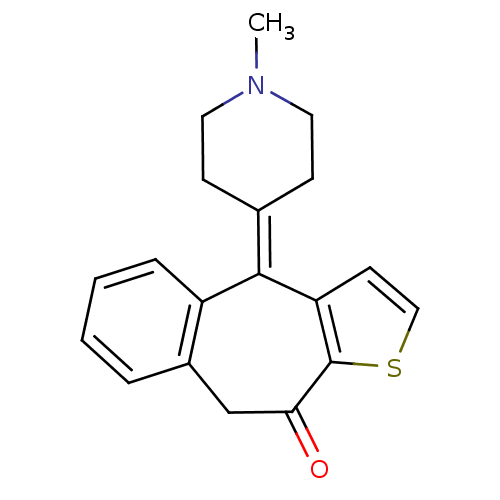 ((Z)-2-butenedioate;10-(1-methyl-4-piperidinylidene...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | US Patent | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BRIDGE PHARMA, INC. US Patent | Assay Description The specific binding of the radioactive ligand to the receptor was defined as the difference between total binding and nonspecific binding, determine... | US Patent US9333199 (2016) BindingDB Entry DOI: 10.7270/Q2639NMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091817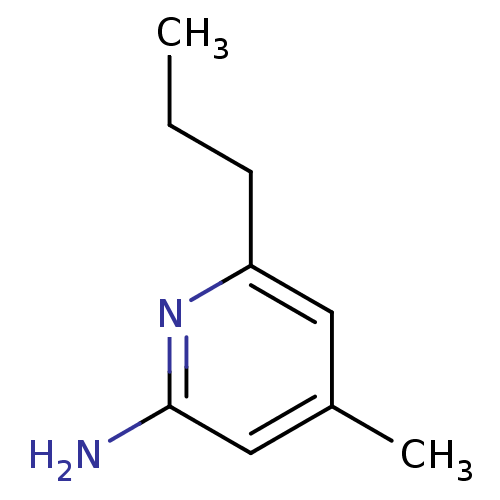 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36395 (5-Fluoro-2-thiophen-2-yl-1,2-dihydroquinazolin-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148168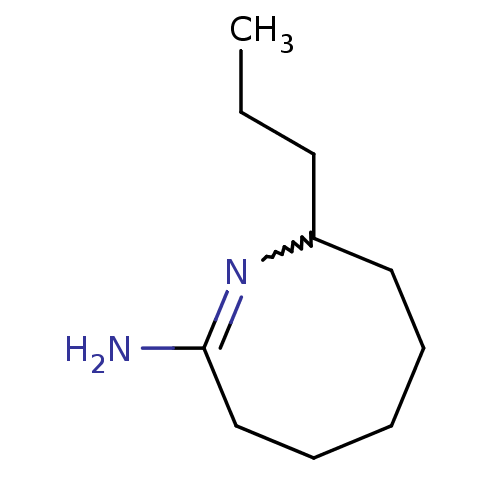 ((E)-8-Propyl-3,4,5,6,7,8-hexahydro-azocin-2-ylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36396 (CD24894148 | N-[2-(4-Amino-5,8-difluoro-1,2-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50124535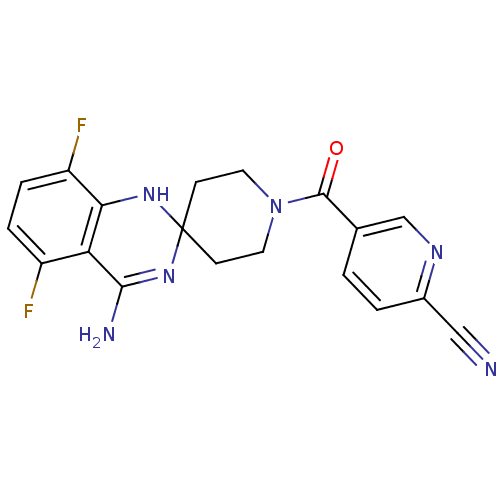 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50124535 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36401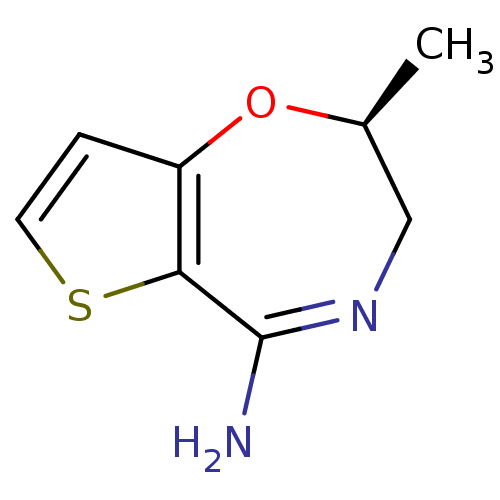 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148169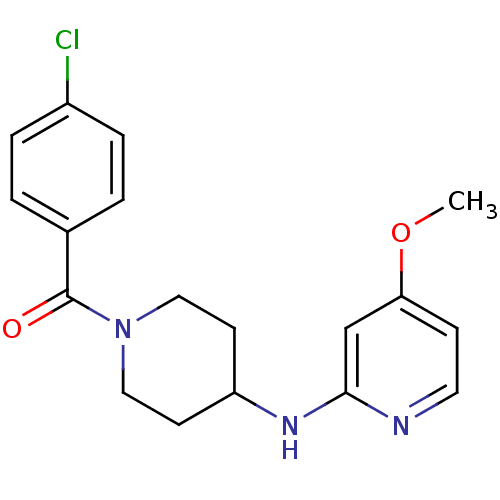 ((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50086467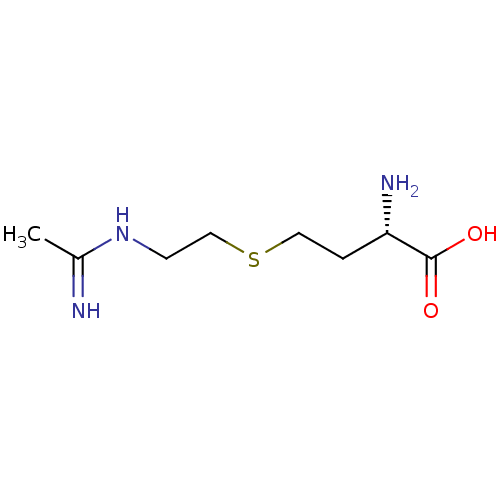 ((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148167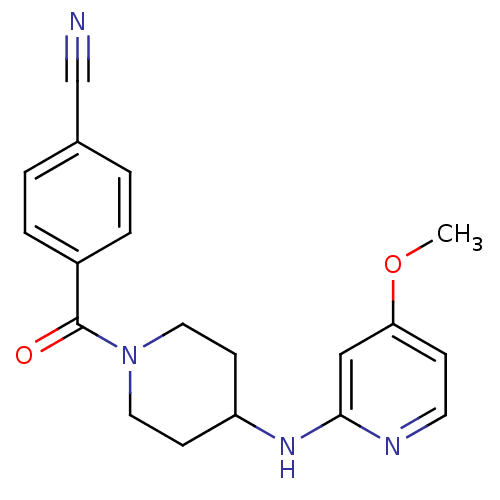 (4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148167 (4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36401 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36401 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148164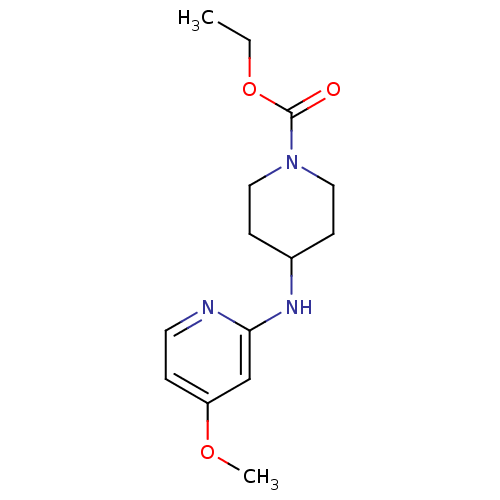 (4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148164 (4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091800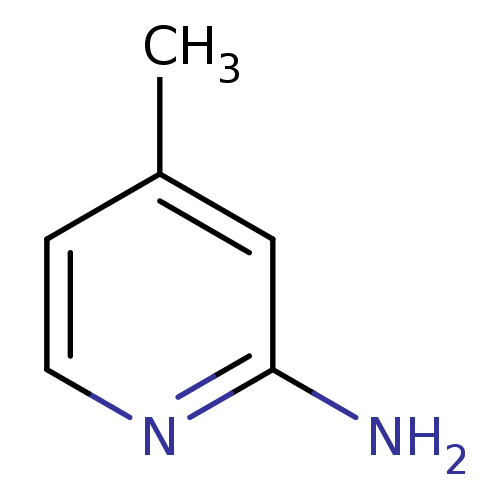 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36399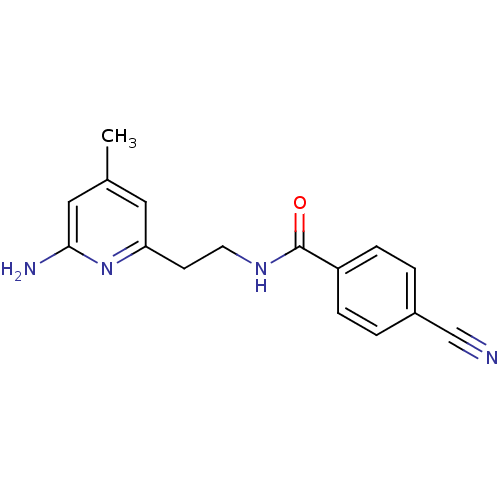 (CID10221335 | N-[2-(6-Amino-4-methylpyridin-2-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36397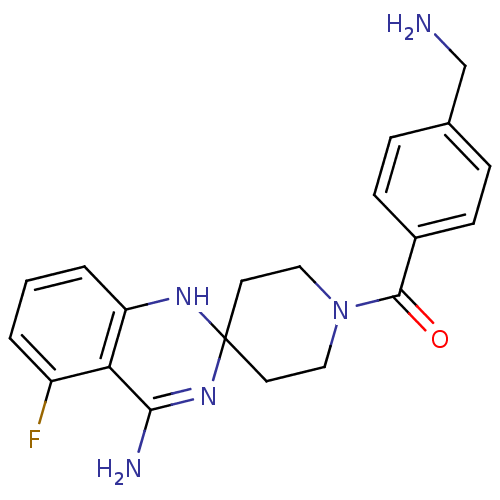 ((4-Amino-5-fluorospiro[1H-quinazoline-2,4'-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50063300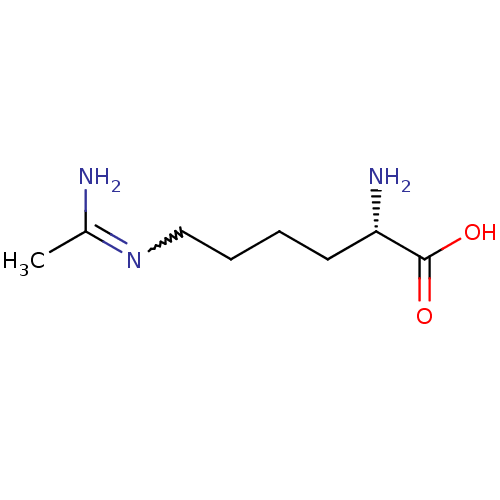 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50148168 ((E)-8-Propyl-3,4,5,6,7,8-hexahydro-azocin-2-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148162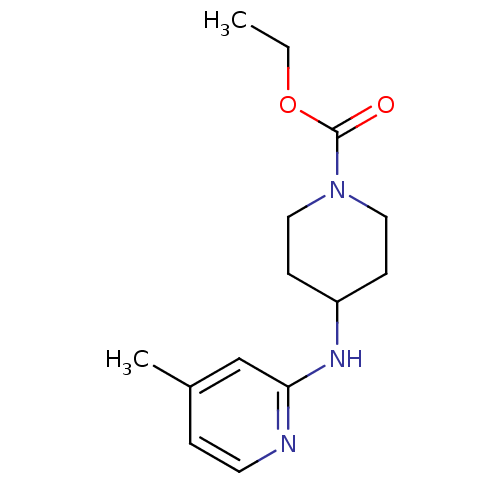 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148162 (4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36403 ((3R)-3-(1,2,3,4-Tetrahydroisoquinolin-7-yloxymethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148163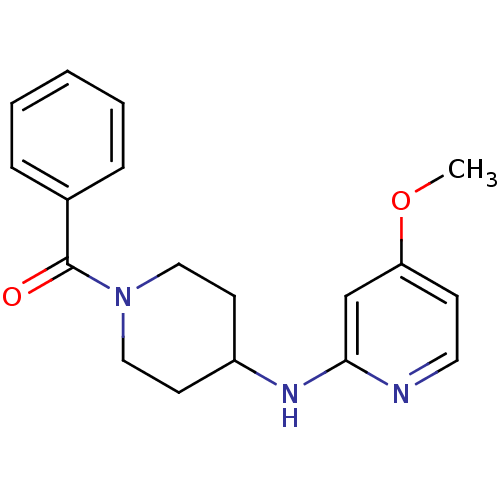 (CHEMBL112747 | [4-(4-Methoxy-pyridin-2-ylamino)-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human Inducible nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36400 (CID24894151 | Ethyl 4-[(4-chloropyridin-2-yl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50124535 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36399 (CID10221335 | N-[2-(6-Amino-4-methylpyridin-2-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50124535 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |