

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289953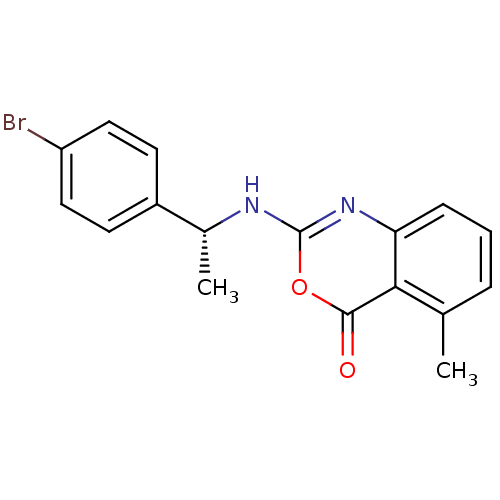 (2-[1-(4-Bromo-phenyl)-ethylamino]-5-methyl-benzo[d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289955 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289952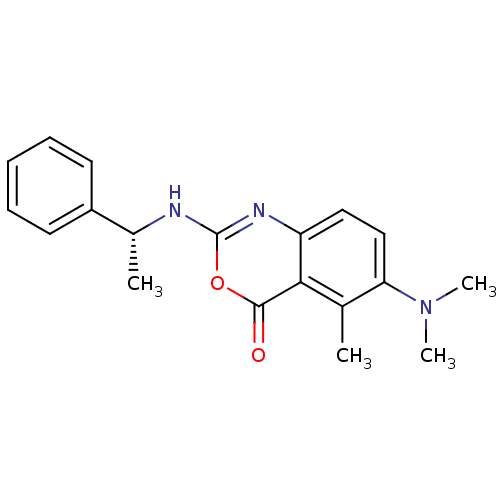 (6-Dimethylamino-5-methyl-2-(1-phenyl-ethylamino)-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289954 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289954 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289953 (2-[1-(4-Bromo-phenyl)-ethylamino]-5-methyl-benzo[d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289955 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289952 (6-Dimethylamino-5-methyl-2-(1-phenyl-ethylamino)-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289950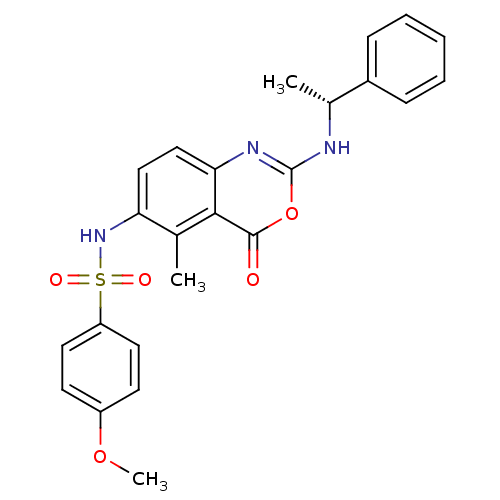 (4-Methoxy-N-[5-methyl-4-oxo-2-(1-phenyl-ethylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289953 (2-[1-(4-Bromo-phenyl)-ethylamino]-5-methyl-benzo[d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289956 (CHEMBL303117 | Pyridine-2-carboxylic acid [5-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289954 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289951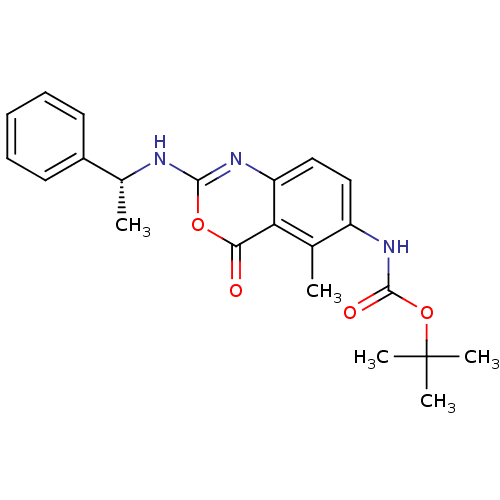 (CHEMBL63952 | [5-Methyl-4-oxo-2-(1-phenyl-ethylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289955 (5-Methyl-2-(1-phenyl-ethylamino)-benzo[d][1,3]oxaz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289948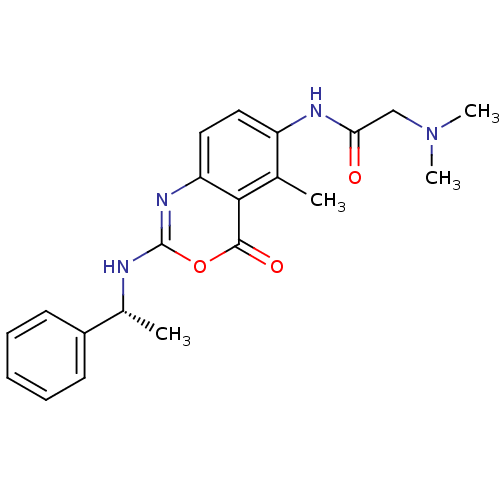 (2-Dimethylamino-N-[5-methyl-4-oxo-2-(1-phenyl-ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50289949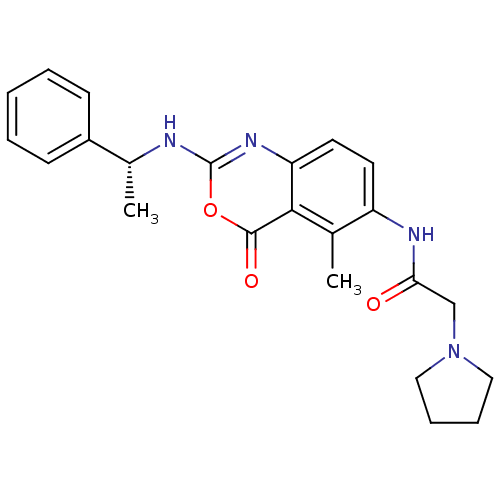 (CHEMBL303802 | N-[5-Methyl-4-oxo-2-(1-phenyl-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289948 (2-Dimethylamino-N-[5-methyl-4-oxo-2-(1-phenyl-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289952 (6-Dimethylamino-5-methyl-2-(1-phenyl-ethylamino)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289950 (4-Methoxy-N-[5-methyl-4-oxo-2-(1-phenyl-ethylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human cytomegalovirus protease variant expressing a beta galactosidase reporter gene | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289951 (CHEMBL63952 | [5-Methyl-4-oxo-2-(1-phenyl-ethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50289951 (CHEMBL63952 | [5-Methyl-4-oxo-2-(1-phenyl-ethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against human chymotrypsin | Bioorg Med Chem Lett 7: 2105-2108 (1997) Article DOI: 10.1016/S0960-894X(97)00368-5 BindingDB Entry DOI: 10.7270/Q2RR1Z6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||