 Found 225 hits with Last Name = 'abraham' and Initial = 'wm'
Found 225 hits with Last Name = 'abraham' and Initial = 'wm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205275
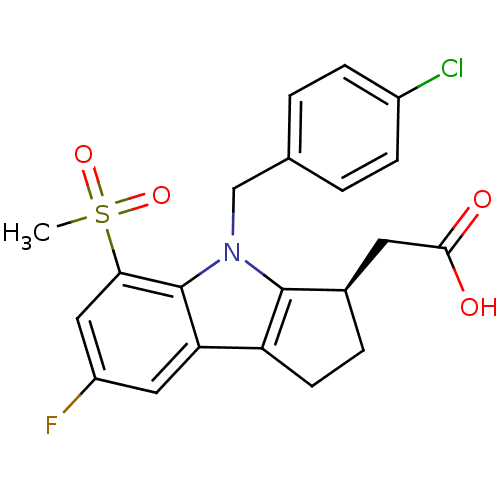
(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205274
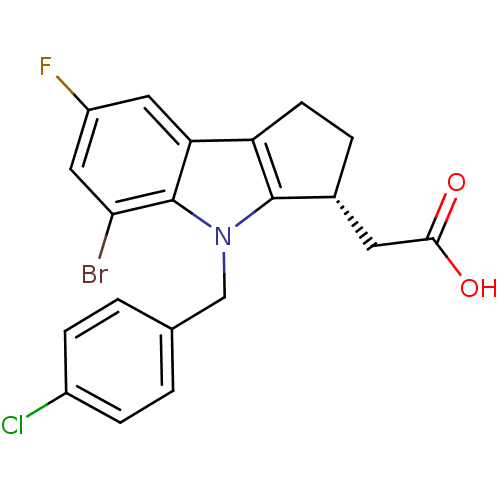
(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205276
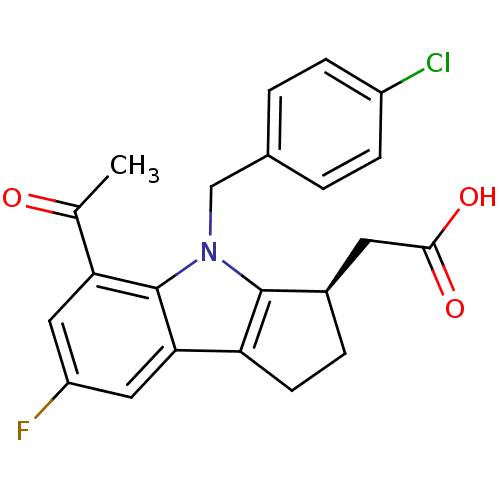
(CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CC(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28)/t14-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205274

(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184217
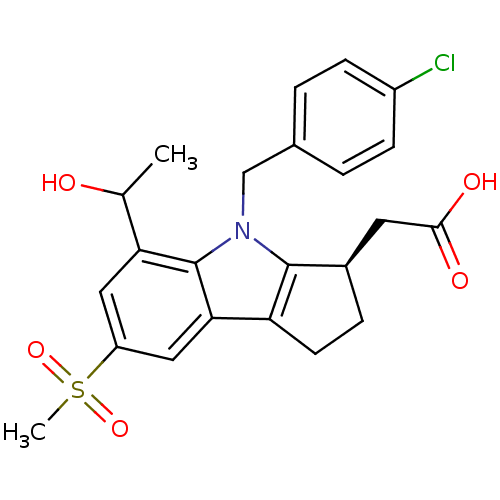
(2-((R)-4-(4-chlorobenzyl)-5-(1-hydroxyethyl)-7-(me...)Show SMILES CC(O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O Show InChI InChI=1S/C23H24ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,13,15,26H,5,8-9,12H2,1-2H3,(H,27,28)/t13?,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184212
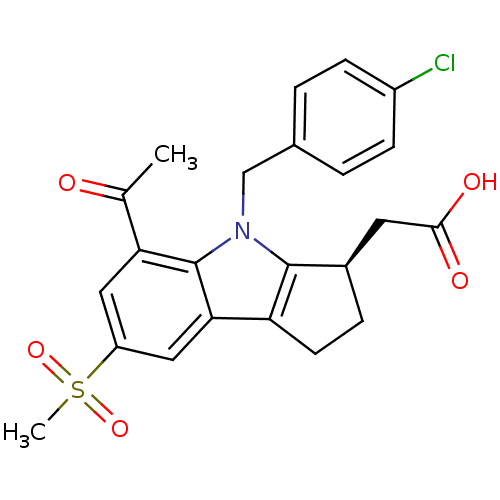
((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...)Show SMILES CC(=O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,15H,5,8-9,12H2,1-2H3,(H,27,28)/t15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205275

(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131975

(1-(2-Methylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C28H35N7O3S/c1-31-21(17-18-9-3-2-4-10-18)27(38)35-16-8-13-22(35)25(37)33-20(12-7-15-32-28(29)30)24(36)26-34-19-11-5-6-14-23(19)39-26/h2-6,9-11,14,20-22,31H,7-8,12-13,15-17H2,1H3,(H,33,37)(H4,29,30,32)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131980

(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES CC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,6,9,14-15H,4-5,7-8,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131975

(1-(2-Methylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C28H35N7O3S/c1-31-21(17-18-9-3-2-4-10-18)27(38)35-16-8-13-22(35)25(37)33-20(12-7-15-32-28(29)30)24(36)26-34-19-11-5-6-14-23(19)39-26/h2-6,9-11,14,20-22,31H,7-8,12-13,15-17H2,1H3,(H,33,37)(H4,29,30,32)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131982
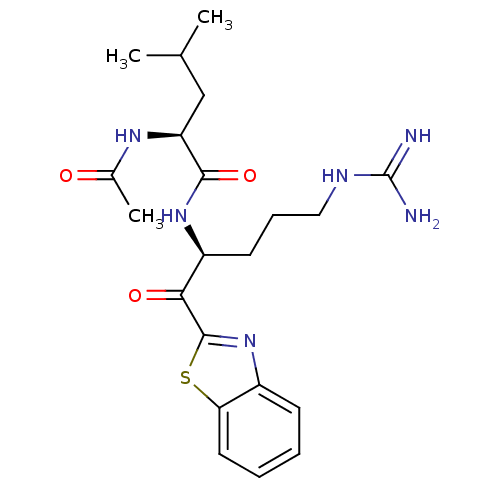
(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C21H30N6O3S/c1-12(2)11-16(25-13(3)28)19(30)26-15(8-6-10-24-21(22)23)18(29)20-27-14-7-4-5-9-17(14)31-20/h4-5,7,9,12,15-16H,6,8,10-11H2,1-3H3,(H,25,28)(H,26,30)(H4,22,23,24)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205278
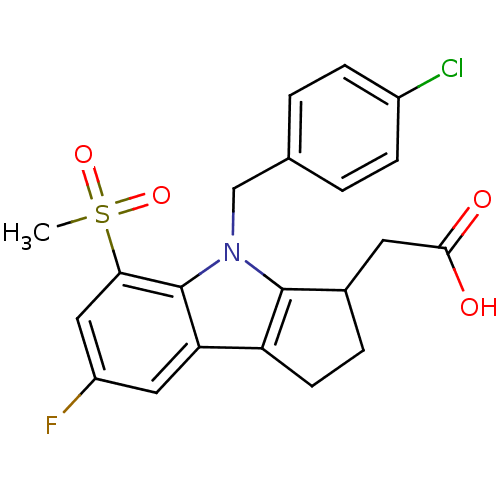
(2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfonyl)-...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131989

(1-Acetyl-2,5-dihydro-1H-pyrrole-2-carboxylic acid ...)Show SMILES CC(=O)N1CC=C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |c:5| Show InChI InChI=1S/C20H24N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,5-6,8-9,14-15H,4,7,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205277
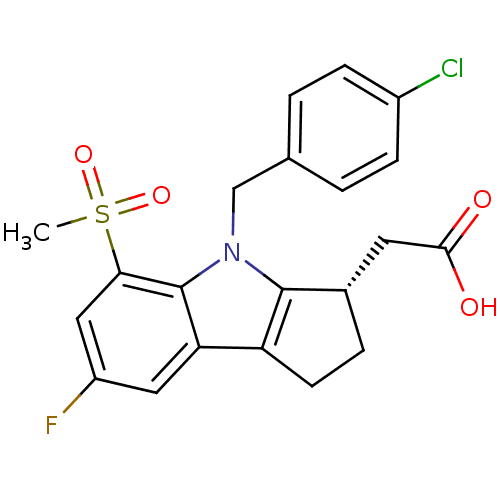
((S)-2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfon...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131983

(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)Show SMILES CC(=O)N1CC(=O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H24N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,14-15H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205276

(CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CC(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131985
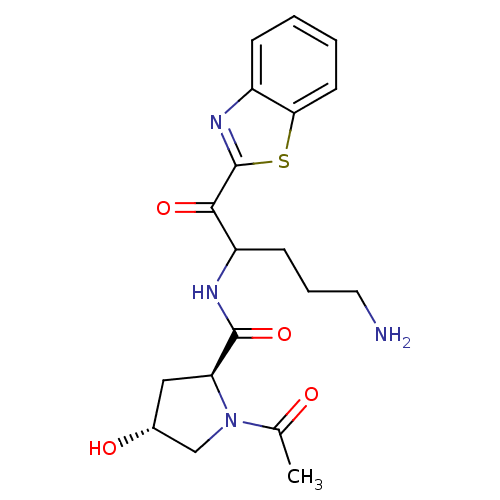
(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)NC(CCCN)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H24N4O4S/c1-11(24)23-10-12(25)9-15(23)18(27)21-14(6-4-8-20)17(26)19-22-13-5-2-3-7-16(13)28-19/h2-3,5,7,12,14-15,25H,4,6,8-10,20H2,1H3,(H,21,27)/t12-,14?,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131980

(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES CC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,6,9,14-15H,4-5,7-8,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131984
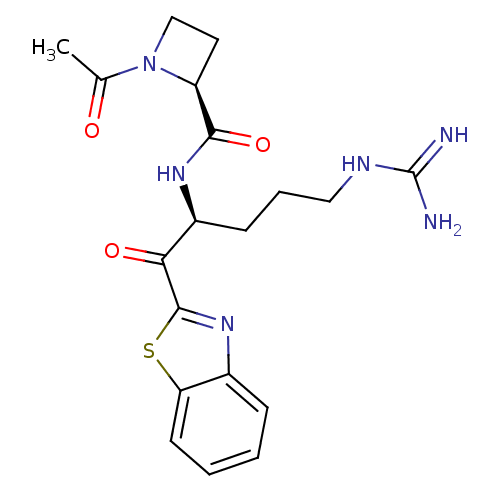
(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)Show SMILES CC(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H24N6O3S/c1-11(26)25-10-8-14(25)17(28)23-13(6-4-9-22-19(20)21)16(27)18-24-12-5-2-3-7-15(12)29-18/h2-3,5,7,13-14H,4,6,8-10H2,1H3,(H,23,28)(H4,20,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131979

(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)Show SMILES NC(=N)NCCCC(NC(=O)C1CCCC1)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H25N5O2S/c20-19(21)22-11-5-9-14(23-17(26)12-6-1-2-7-12)16(25)18-24-13-8-3-4-10-15(13)27-18/h3-4,8,10,12,14H,1-2,5-7,9,11H2,(H,23,26)(H4,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131988
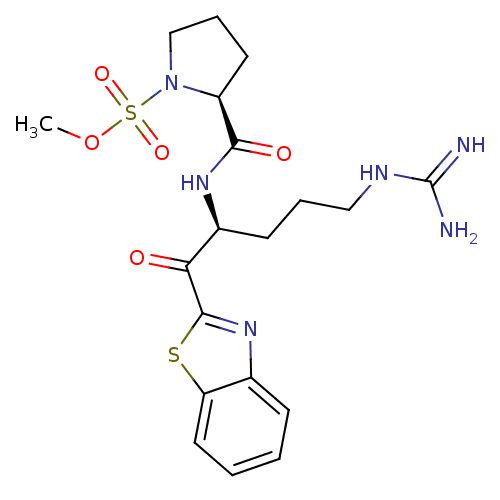
(2-[1-(Benzothiazole-2-carbonyl)-4-guanidino-butylc...)Show SMILES COS(=O)(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H26N6O5S2/c1-30-32(28,29)25-11-5-8-14(25)17(27)23-13(7-4-10-22-19(20)21)16(26)18-24-12-6-2-3-9-15(12)31-18/h2-3,6,9,13-14H,4-5,7-8,10-11H2,1H3,(H,23,27)(H4,20,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131989

(1-Acetyl-2,5-dihydro-1H-pyrrole-2-carboxylic acid ...)Show SMILES CC(=O)N1CC=C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |c:5| Show InChI InChI=1S/C20H24N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,5-6,8-9,14-15H,4,7,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131988

(2-[1-(Benzothiazole-2-carbonyl)-4-guanidino-butylc...)Show SMILES COS(=O)(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H26N6O5S2/c1-30-32(28,29)25-11-5-8-14(25)17(27)23-13(7-4-10-22-19(20)21)16(26)18-24-12-6-2-3-9-15(12)31-18/h2-3,6,9,13-14H,4-5,7-8,10-11H2,1H3,(H,23,27)(H4,20,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131982

(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C21H30N6O3S/c1-12(2)11-16(25-13(3)28)19(30)26-15(8-6-10-24-21(22)23)18(29)20-27-14-7-4-5-9-17(14)31-20/h4-5,7,9,12,15-16H,6,8,10-11H2,1-3H3,(H,25,28)(H,26,30)(H4,22,23,24)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131984

(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)Show SMILES CC(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H24N6O3S/c1-11(26)25-10-8-14(25)17(28)23-13(6-4-9-22-19(20)21)16(27)18-24-12-5-2-3-7-15(12)29-18/h2-3,5,7,13-14H,4,6,8-10H2,1H3,(H,23,28)(H4,20,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131983

(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)Show SMILES CC(=O)N1CC(=O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H24N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,14-15H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131974
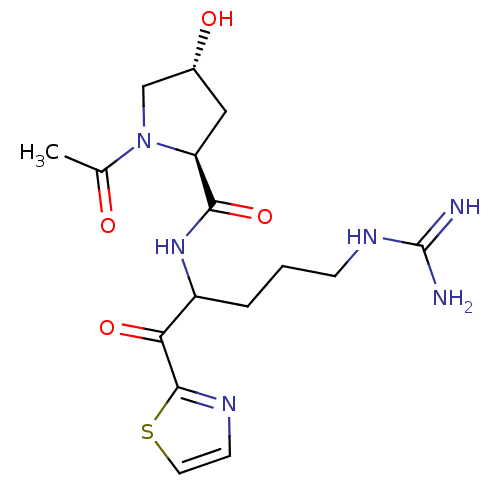
(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C16H24N6O4S/c1-9(23)22-8-10(24)7-12(22)14(26)21-11(3-2-4-20-16(17)18)13(25)15-19-5-6-27-15/h5-6,10-12,24H,2-4,7-8H2,1H3,(H,21,26)(H4,17,18,20)/t10-,11?,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131979

(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)Show SMILES NC(=N)NCCCC(NC(=O)C1CCCC1)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H25N5O2S/c20-19(21)22-11-5-9-14(23-17(26)12-6-1-2-7-12)16(25)18-24-13-8-3-4-10-15(13)27-18/h3-4,8,10,12,14H,1-2,5-7,9,11H2,(H,23,26)(H4,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131985

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)NC(CCCN)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H24N4O4S/c1-11(24)23-10-12(25)9-15(23)18(27)21-14(6-4-8-20)17(26)19-22-13-5-2-3-7-16(13)28-19/h2-3,5,7,12,14-15,25H,4,6,8-10,20H2,1H3,(H,21,27)/t12-,14?,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM16127
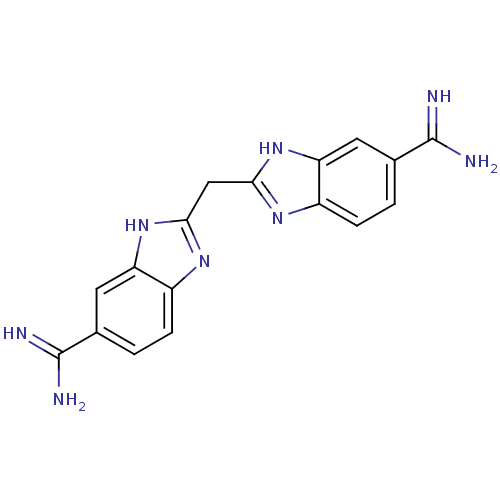
(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50205275

(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM16127

(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131978
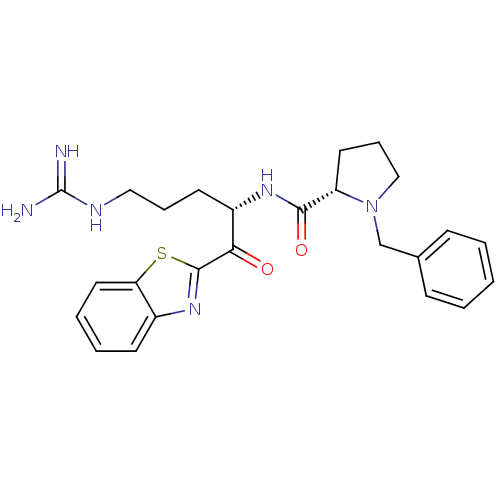
(1-Benzyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H]1CCCN1Cc1ccccc1)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C25H30N6O2S/c26-25(27)28-14-6-11-19(22(32)24-30-18-10-4-5-13-21(18)34-24)29-23(33)20-12-7-15-31(20)16-17-8-2-1-3-9-17/h1-5,8-10,13,19-20H,6-7,11-12,14-16H2,(H,29,33)(H4,26,27,28)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131974

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C16H24N6O4S/c1-9(23)22-8-10(24)7-12(22)14(26)21-11(3-2-4-20-16(17)18)13(25)15-19-5-6-27-15/h5-6,10-12,24H,2-4,7-8H2,1H3,(H,21,26)(H4,17,18,20)/t10-,11?,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50184212

((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...)Show SMILES CC(=O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,15H,5,8-9,12H2,1-2H3,(H,27,28)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131978

(1-Benzyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H]1CCCN1Cc1ccccc1)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C25H30N6O2S/c26-25(27)28-14-6-11-19(22(32)24-30-18-10-4-5-13-21(18)34-24)29-23(33)20-12-7-15-31(20)16-17-8-2-1-3-9-17/h1-5,8-10,13,19-20H,6-7,11-12,14-16H2,(H,29,33)(H4,26,27,28)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131981
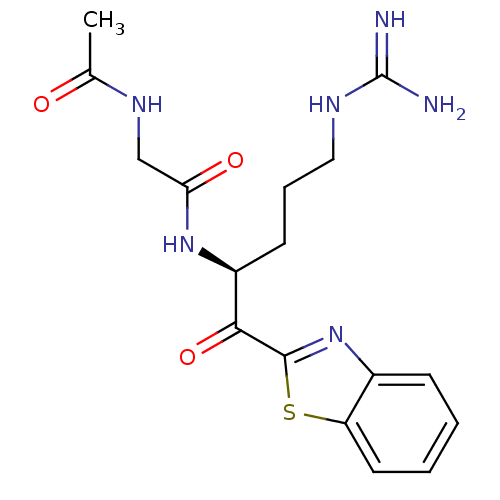
(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)Show SMILES CC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C17H22N6O3S/c1-10(24)21-9-14(25)22-12(6-4-8-20-17(18)19)15(26)16-23-11-5-2-3-7-13(11)27-16/h2-3,5,7,12H,4,6,8-9H2,1H3,(H,21,24)(H,22,25)(H4,18,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50205276

(CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CC(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131987
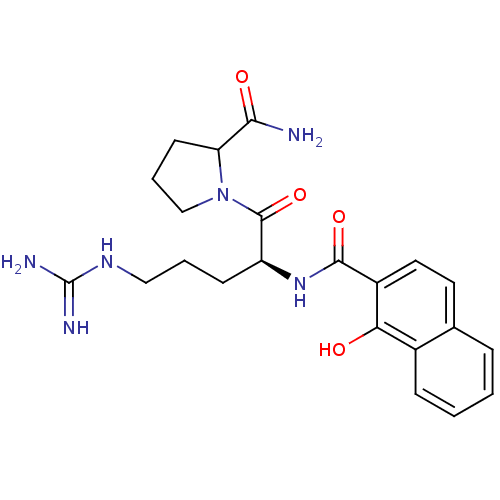
(1-{5-Guanidino-2-[(1-hydroxy-naphthalene-2-carbony...)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc2ccccc2c1O)C(=O)N1CCCC1C(N)=O Show InChI InChI=1S/C22H28N6O4/c23-19(30)17-8-4-12-28(17)21(32)16(7-3-11-26-22(24)25)27-20(31)15-10-9-13-5-1-2-6-14(13)18(15)29/h1-2,5-6,9-10,16-17,29H,3-4,7-8,11-12H2,(H2,23,30)(H,27,31)(H4,24,25,26)/t16-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50184217

(2-((R)-4-(4-chlorobenzyl)-5-(1-hydroxyethyl)-7-(me...)Show SMILES CC(O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O Show InChI InChI=1S/C23H24ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,13,15,26H,5,8-9,12H2,1-2H3,(H,27,28)/t13?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50205274

(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human IP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50205274

(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50205274

(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131981

(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)Show SMILES CC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C17H22N6O3S/c1-10(24)21-9-14(25)22-12(6-4-8-20-17(18)19)15(26)16-23-11-5-2-3-7-13(11)27-16/h2-3,5,7,12H,4,6,8-9H2,1H3,(H,21,24)(H,22,25)(H4,18,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data