

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15067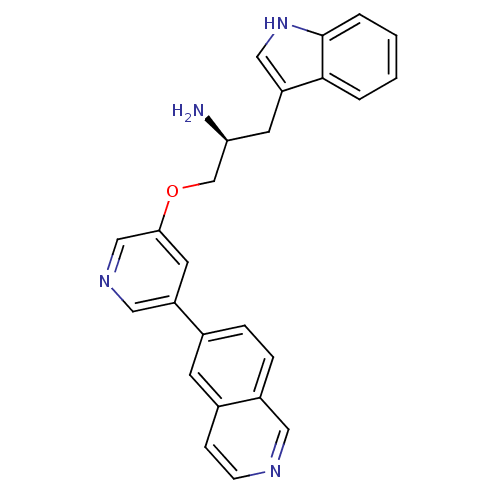 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050933 ((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050935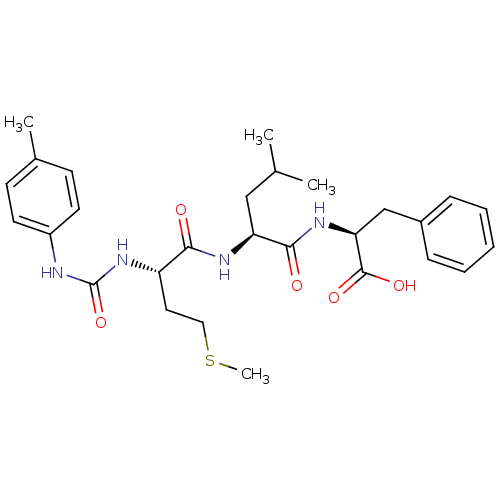 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050929 (2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM15067 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15067 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15022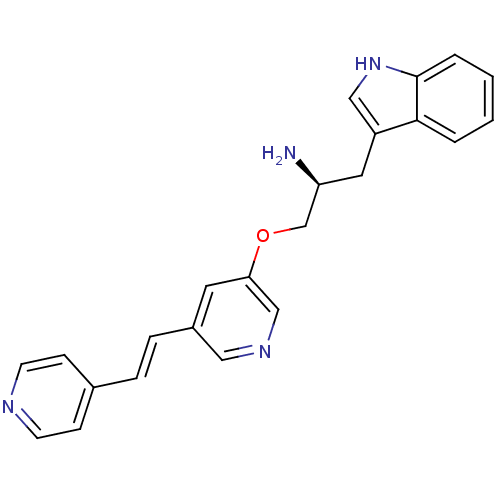 (3-[(2S)-2-amino-3-({5-[(E)-2-(pyridin-4-yl)ethenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937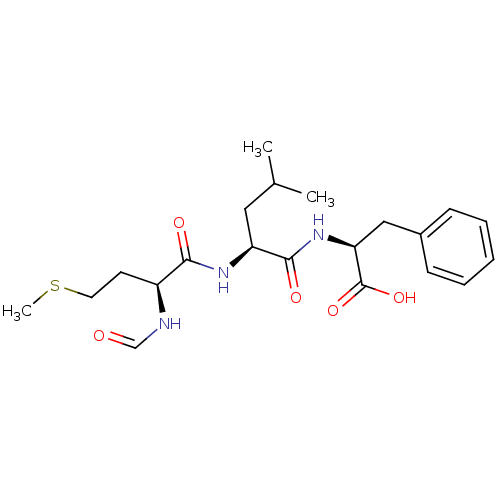 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928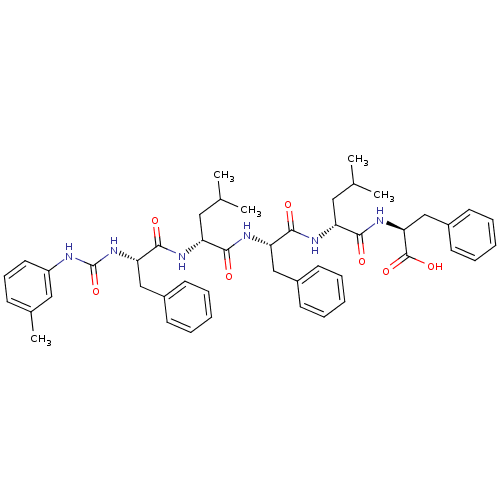 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15068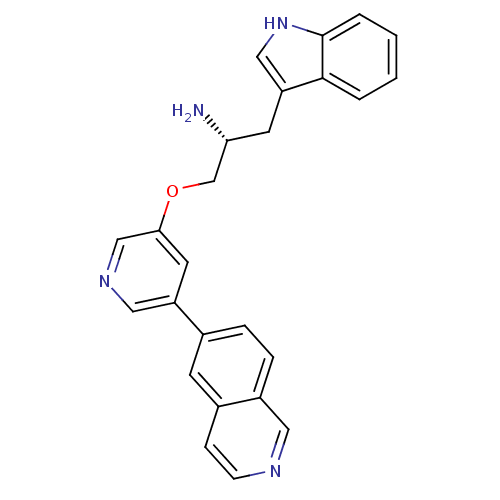 (13b (R-) | 6-{5-[(2R)-2-amino-3-(1H-indol-3-yl)pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050946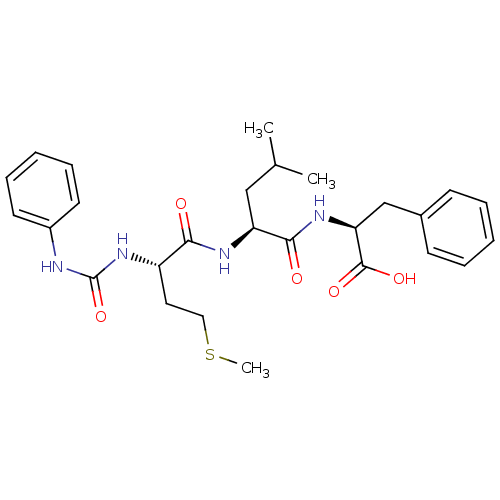 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15067 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM15022 (3-[(2S)-2-amino-3-({5-[(E)-2-(pyridin-4-yl)ethenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4851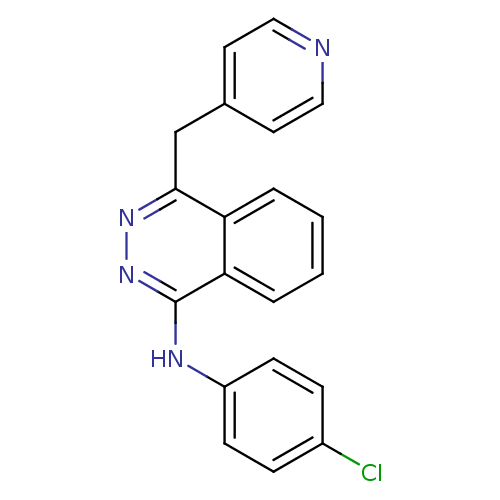 ((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of VEGFR2 | Nat Chem Biol 2: 265-73 (2006) Article DOI: 10.1038/nchembio778 BindingDB Entry DOI: 10.7270/Q2TT4R56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4810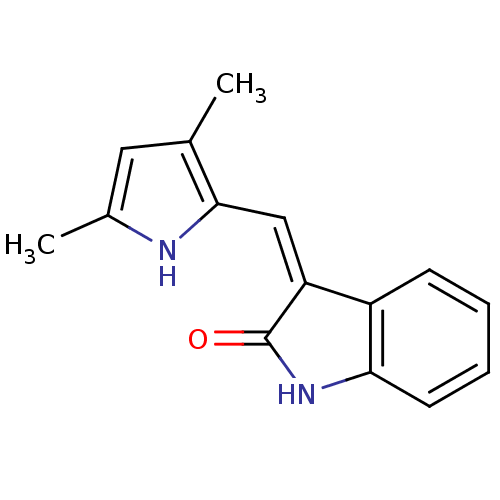 ((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Nat Chem Biol 2: 265-73 (2006) Article DOI: 10.1038/nchembio778 BindingDB Entry DOI: 10.7270/Q2TT4R56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050926 ((S)-2-((R)-4-Methyl-2-{(S)-2-[(R)-4-methyl-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050939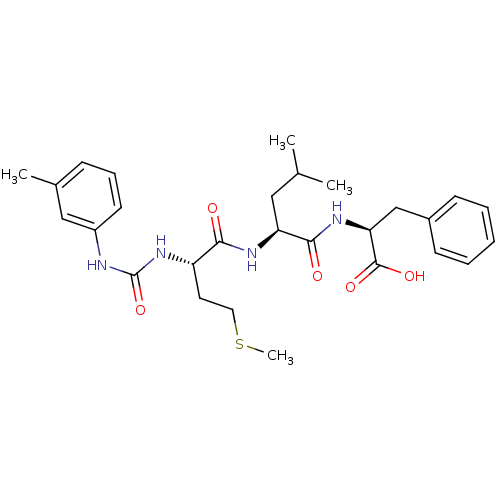 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15066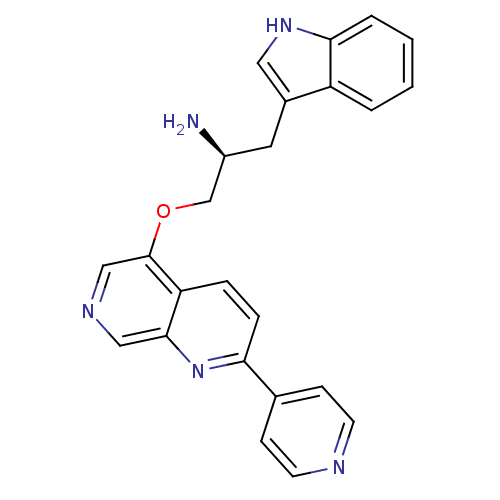 (5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-(pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15064 (N-[(2S)-2-amino-3-(1H-indol-3-yl)propyl]-5-(isoqui...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15023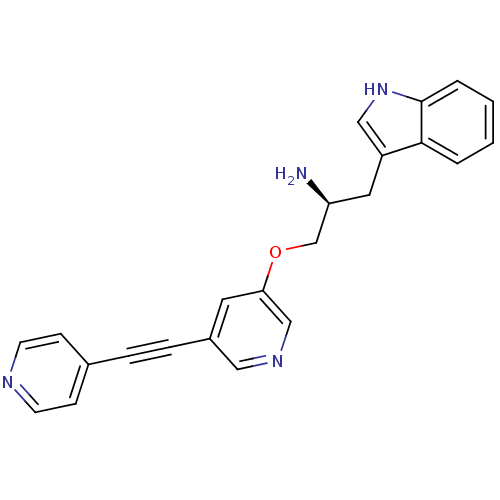 (3-[(2S)-2-amino-3-({5-[2-(pyridin-4-yl)ethynyl]pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4810 ((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of VEGFR2 | Nat Chem Biol 2: 265-73 (2006) Article DOI: 10.1038/nchembio778 BindingDB Entry DOI: 10.7270/Q2TT4R56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15050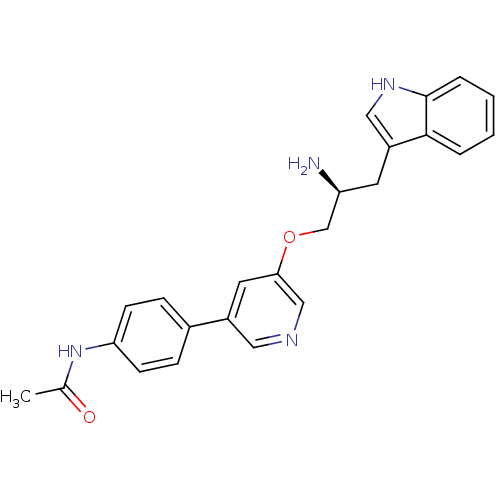 (N-(4-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15022 (3-[(2S)-2-amino-3-({5-[(E)-2-(pyridin-4-yl)ethenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15037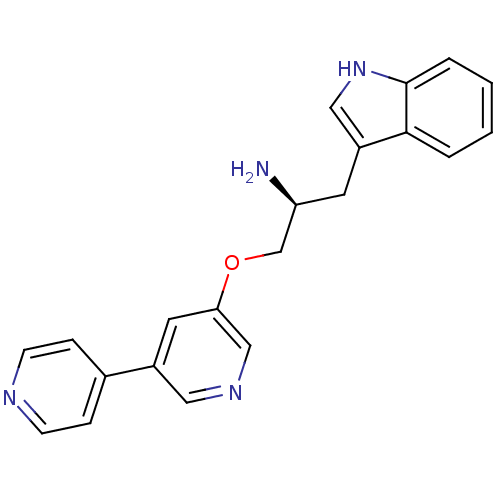 (3-[(2S)-2-amino-3-{[5-(pyridin-4-yl)pyridin-3-yl]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM15067 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15065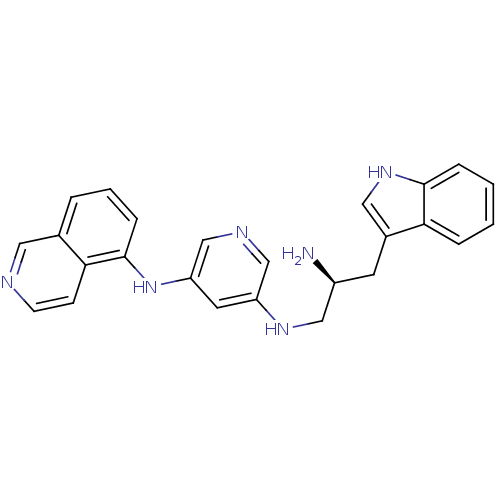 (3-N-[(2S)-2-amino-3-(1H-indol-3-yl)propyl]-5-N-(is...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050927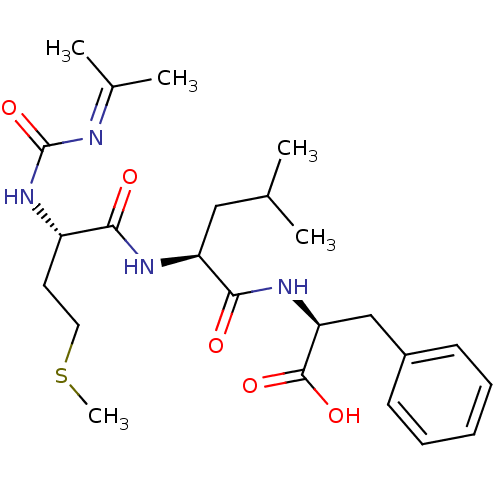 ((S)-2-{(S)-2-[(S)-2-(3-Isopropenyl-ureido)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050934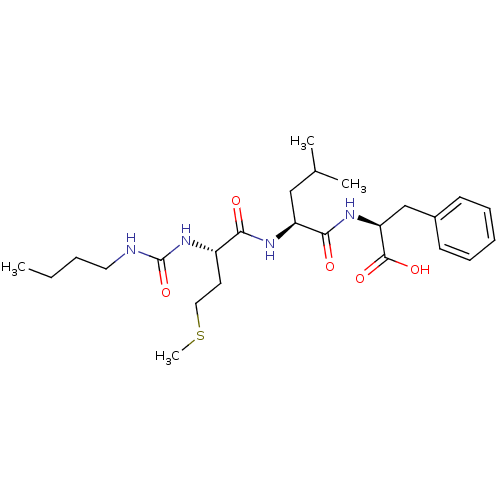 ((S)-2-{(S)-2-[(S)-2-(3-Butyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050931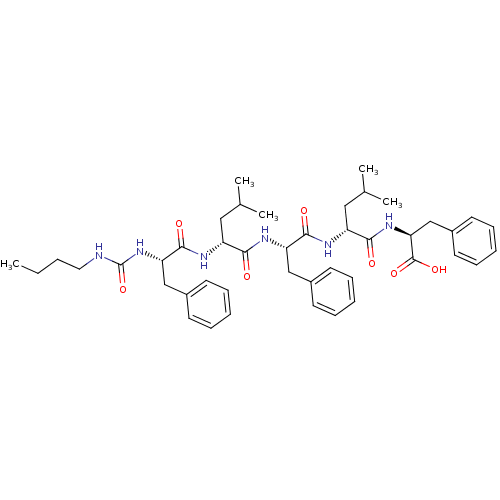 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Butyl-ureido)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15062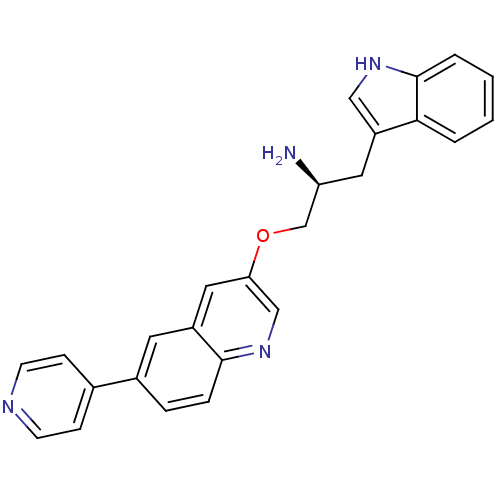 (3-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-6-(pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15022 (3-[(2S)-2-amino-3-({5-[(E)-2-(pyridin-4-yl)ethenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 354 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4851 ((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Nat Chem Biol 2: 265-73 (2006) Article DOI: 10.1038/nchembio778 BindingDB Entry DOI: 10.7270/Q2TT4R56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050931 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Butyl-ureido)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050940 ((S)-2-{(S)-2-[(S)-2-(3-Benzyl-ureido)-4-methylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15021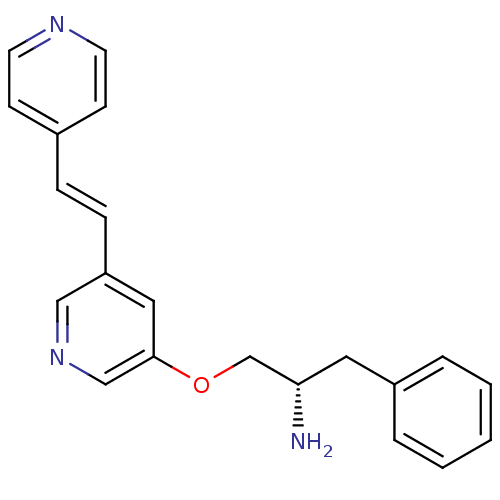 (3-[(2S)-2-amino-3-phenylpropoxy]-5-[(E)-2-(pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 2000-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.065 BindingDB Entry DOI: 10.7270/Q2765CKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050940 ((S)-2-{(S)-2-[(S)-2-(3-Benzyl-ureido)-4-methylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050926 ((S)-2-((R)-4-Methyl-2-{(S)-2-[(R)-4-methyl-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |