 Found 29 hits with Last Name = 'abt' and Initial = 'j'
Found 29 hits with Last Name = 'abt' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046071
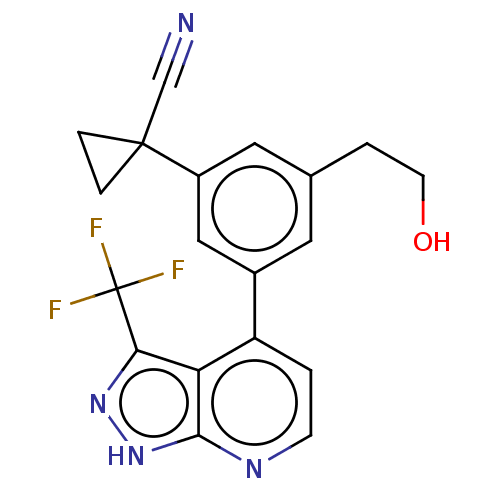
(CHEMBL3310277)Show SMILES OCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H15F3N4O/c20-19(21,22)16-15-14(1-5-24-17(15)26-25-16)12-7-11(2-6-27)8-13(9-12)18(10-23)3-4-18/h1,5,7-9,27H,2-4,6H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046102

(CHEMBL3310293)Show SMILES OCC(O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046097
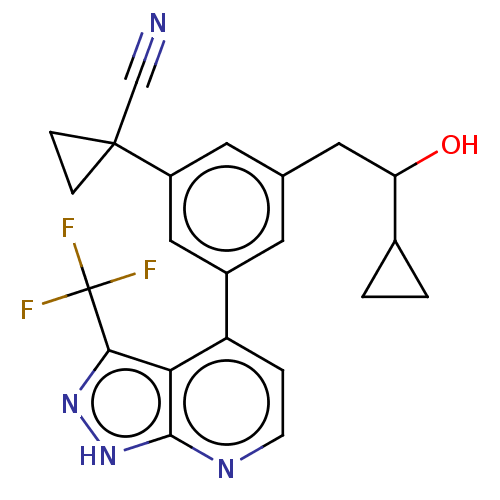
(CHEMBL3310288)Show SMILES OC(Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046066
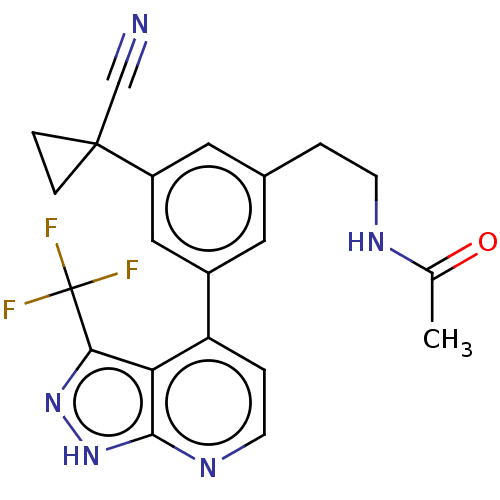
(CHEMBL3310284)Show SMILES CC(=O)NCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C21H18F3N5O/c1-12(30)26-6-2-13-8-14(10-15(9-13)20(11-25)4-5-20)16-3-7-27-19-17(16)18(28-29-19)21(22,23)24/h3,7-10H,2,4-6H2,1H3,(H,26,30)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046095
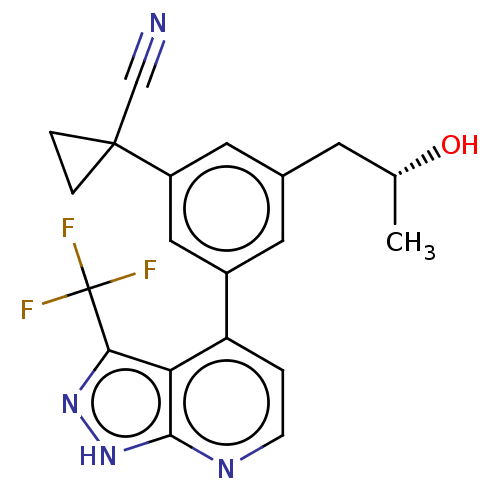
(CHEMBL3310286)Show SMILES C[C@@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50281603

([Hydroxy-((2E,6E)-3,7,11-trimethyl-dodeca-2,6,10-t...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-[#6]P([#8-])(=O)[#6]P([#8-])([#8-])=O Show InChI InChI=1S/C17H32O6P2/c1-15(2)7-5-8-16(3)9-6-10-17(4)11-12-23-13-24(18,19)14-25(20,21)22/h7,9,11H,5-6,8,10,12-14H2,1-4H3,(H,18,19)(H2,20,21,22)/p-3/b16-9-,17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory potency against rat liver microsomal squalene synthase |
Bioorg Med Chem Lett 3: 595-600 (1993)
Article DOI: 10.1016/S0960-894X(01)81236-1
BindingDB Entry DOI: 10.7270/Q25Q4WMR |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046058
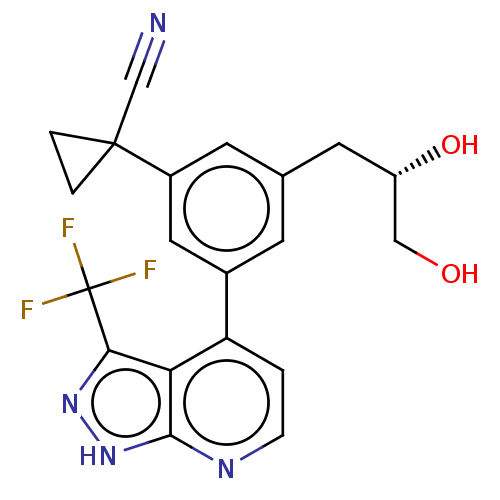
(CHEMBL3310294)Show SMILES OC[C@@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046059
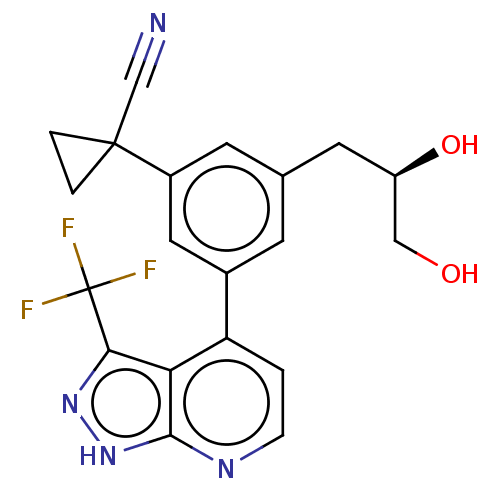
(CHEMBL3310295)Show SMILES OC[C@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046098

(CHEMBL3310289)Show SMILES CCC(O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046069
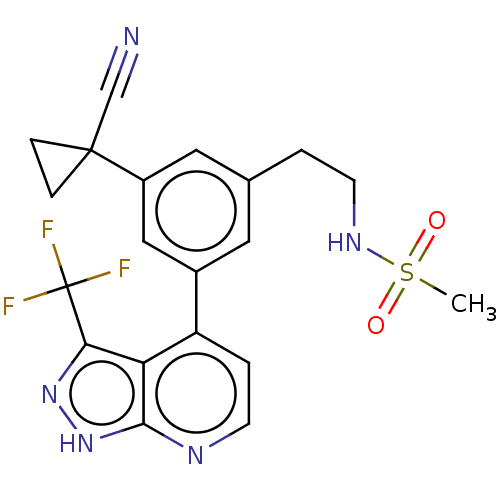
(CHEMBL3310285)Show SMILES CS(=O)(=O)NCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C20H18F3N5O2S/c1-31(29,30)26-7-2-12-8-13(10-14(9-12)19(11-24)4-5-19)15-3-6-25-18-16(15)17(27-28-18)20(21,22)23/h3,6,8-10,26H,2,4-5,7H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046096
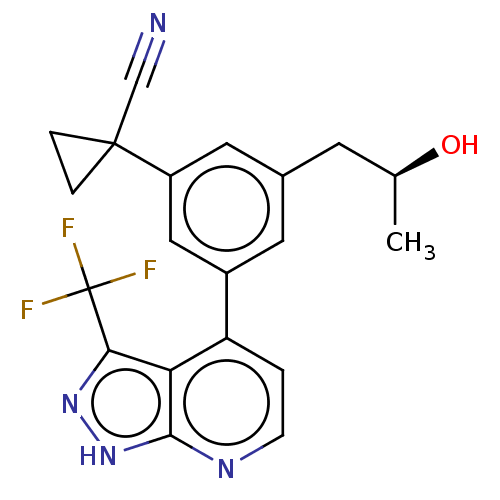
(CHEMBL3310287)Show SMILES C[C@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046103
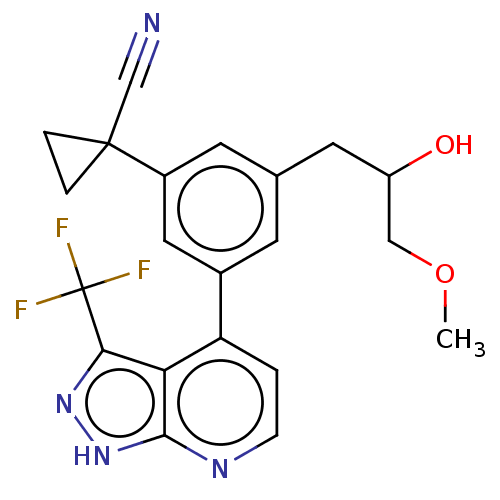
(CHEMBL3310296)Show SMILES COCC(O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046088

(CHEMBL3310278)Show SMILES CCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H15F3N4/c1-2-11-7-12(9-13(8-11)18(10-23)4-5-18)14-3-6-24-17-15(14)16(25-26-17)19(20,21)22/h3,6-9H,2,4-5H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046089

(CHEMBL3310279)Show SMILES FCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H14F4N4/c20-5-1-11-7-12(9-13(8-11)18(10-24)3-4-18)14-2-6-25-17-15(14)16(26-27-17)19(21,22)23/h2,6-9H,1,3-5H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046093

(CHEMBL3310282)Show SMILES CC(=O)OCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12(29)30-7-3-13-8-14(10-15(9-13)20(11-25)4-5-20)16-2-6-26-19-17(16)18(27-28-19)21(22,23)24/h2,6,8-10H,3-5,7H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046092
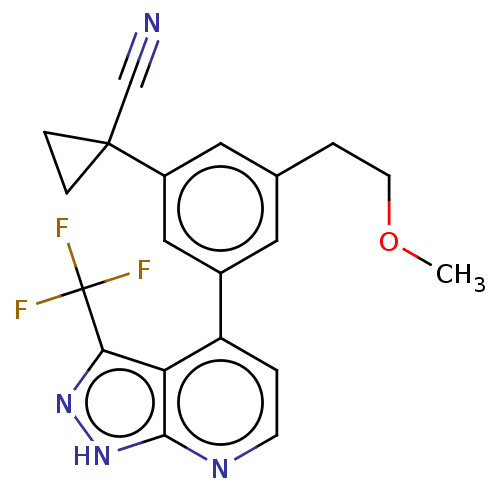
(CHEMBL3310281)Show SMILES COCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C20H17F3N4O/c1-28-7-3-12-8-13(10-14(9-12)19(11-24)4-5-19)15-2-6-25-18-16(15)17(26-27-18)20(21,22)23/h2,6,8-10H,3-5,7H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046094

(CHEMBL3310283)Show SMILES NCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H16F3N5/c20-19(21,22)16-15-14(2-6-25-17(15)27-26-16)12-7-11(1-5-23)8-13(9-12)18(10-24)3-4-18/h2,6-9H,1,3-5,23H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046091
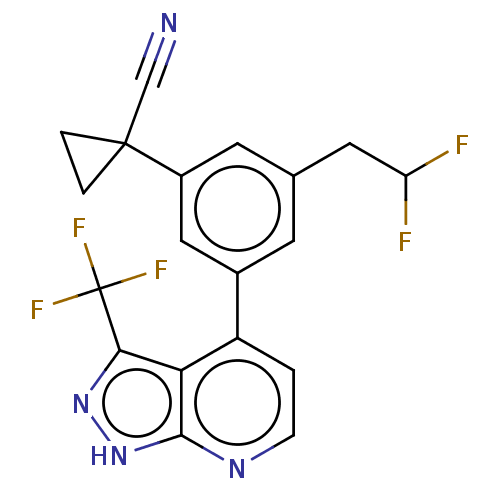
(CHEMBL3310280)Show SMILES FC(F)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H13F5N4/c20-14(21)7-10-5-11(8-12(6-10)18(9-25)2-3-18)13-1-4-26-17-15(13)16(27-28-17)19(22,23)24/h1,4-6,8,14H,2-3,7H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046099
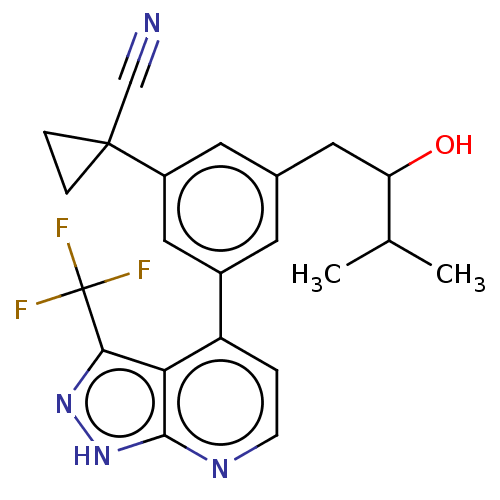
(CHEMBL3310290)Show SMILES CC(C)C(O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046100
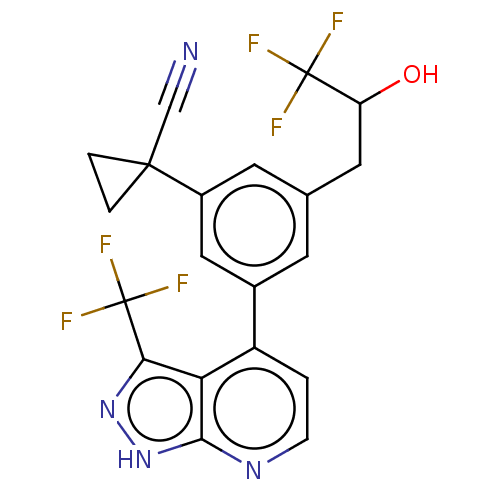
(CHEMBL3310291)Show SMILES OC(Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50046101

(CHEMBL3310292)Show SMILES OC(=O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C19H13F3N4O2/c20-19(21,22)16-15-13(1-4-24-17(15)26-25-16)11-5-10(7-14(27)28)6-12(8-11)18(9-23)2-3-18/h1,4-6,8H,2-3,7H2,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 446 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50046069

(CHEMBL3310285)Show SMILES CS(=O)(=O)NCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C20H18F3N5O2S/c1-31(29,30)26-7-2-12-8-13(10-14(9-12)19(11-24)4-5-19)15-3-6-25-18-16(15)17(27-28-18)20(21,22)23/h3,6,8-10,26H,2,4-5,7H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50046066

(CHEMBL3310284)Show SMILES CC(=O)NCCc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F Show InChI InChI=1S/C21H18F3N5O/c1-12(30)26-6-2-13-8-14(10-15(9-13)20(11-25)4-5-20)16-3-7-27-19-17(16)18(28-29-19)21(22,23)24/h3,7-10H,2,4-6H2,1H3,(H,26,30)(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using dextromethorphan as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50046058

(CHEMBL3310294)Show SMILES OC[C@@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50046059

(CHEMBL3310295)Show SMILES OC[C@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50046058

(CHEMBL3310294)Show SMILES OC[C@@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 using diclofenac as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50046058

(CHEMBL3310294)Show SMILES OC[C@@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using dextromethorphan as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50046059

(CHEMBL3310295)Show SMILES OC[C@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using dextromethorphan as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50046059

(CHEMBL3310295)Show SMILES OC[C@H](O)Cc1cc(cc(c1)C1(CC1)C#N)-c1ccnc2[nH]nc(c12)C(F)(F)F |r| Show InChI InChI=1S/C20H17F3N4O2/c21-20(22,23)17-16-15(1-4-25-18(16)27-26-17)12-5-11(7-14(29)9-28)6-13(8-12)19(10-24)2-3-19/h1,4-6,8,14,28-29H,2-3,7,9H2,(H,25,26,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 using diclofenac as substrate after 10 mins by SPE-MS analysis in presence of NADPH |
Bioorg Med Chem Lett 24: 3398-402 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.082
BindingDB Entry DOI: 10.7270/Q27H1M7X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data