

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187213 (CHEMBL3828586) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108602 (CHEMBL3597942) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108604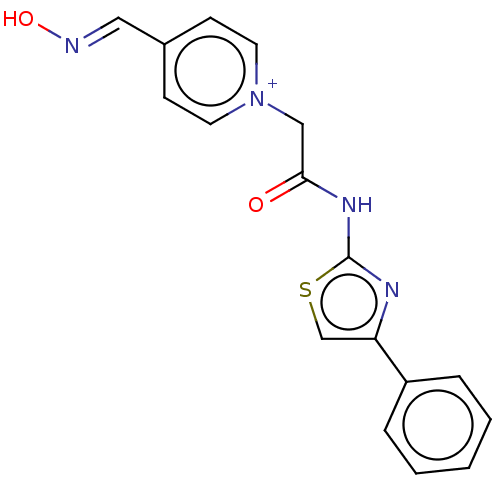 (CHEMBL3597941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780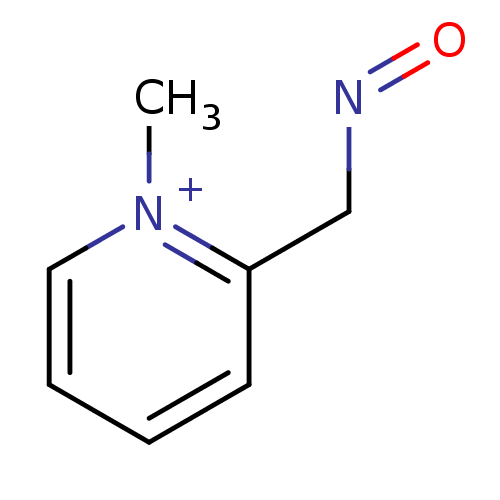 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187214 (CHEMBL3828385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108601 (CHEMBL3597944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187215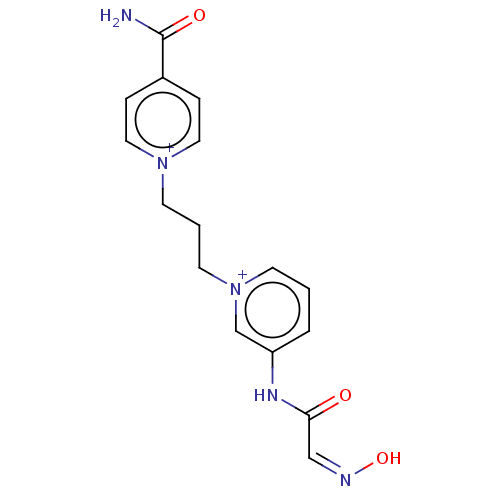 (CHEMBL3828381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187216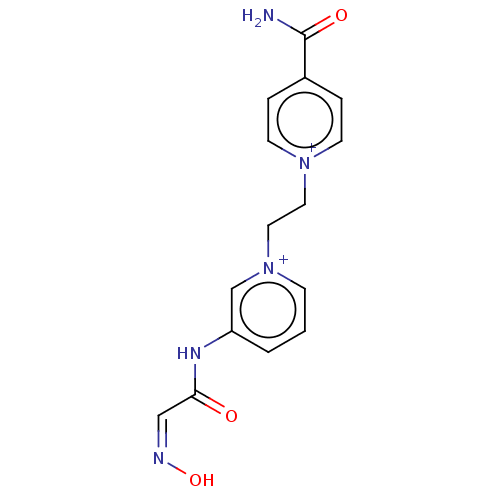 (CHEMBL3827575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108600 (CHEMBL3597946) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108607 (CHEMBL3597939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.36E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108574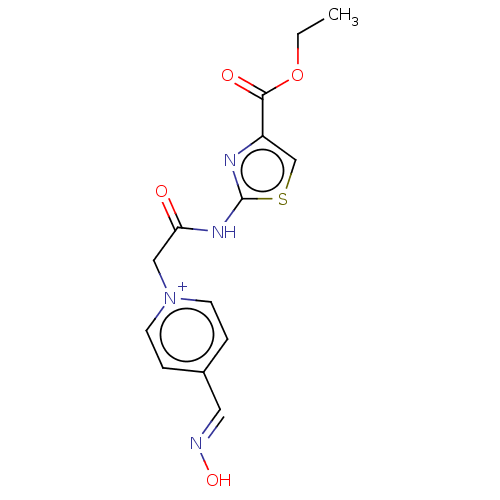 (CHEMBL3597948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase incubated for 60 mins using ATChI substrate by DTNB dye based UV-visible spectrophotometry | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108574 (CHEMBL3597948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108599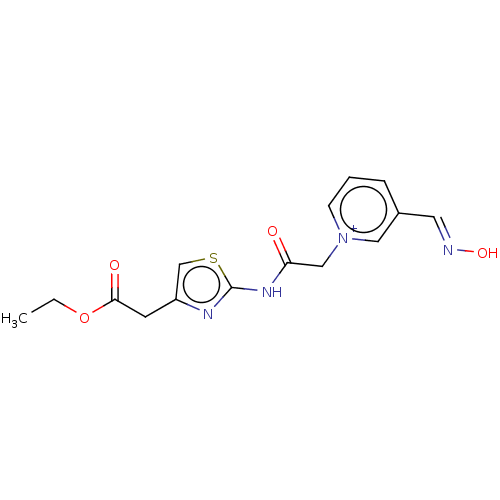 (CHEMBL3597947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108600 (CHEMBL3597946) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.69E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108609 (CHEMBL3597945) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.41E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108601 (CHEMBL3597944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.37E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108602 (CHEMBL3597942) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.75E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108603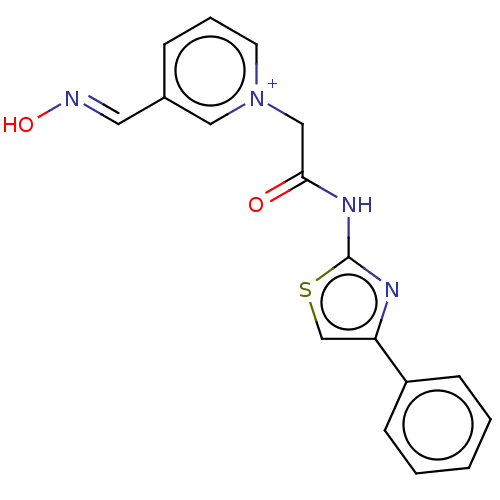 (CHEMBL3596215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.83E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108604 (CHEMBL3597941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.69E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108605 (CHEMBL3597940) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108607 (CHEMBL3597939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005579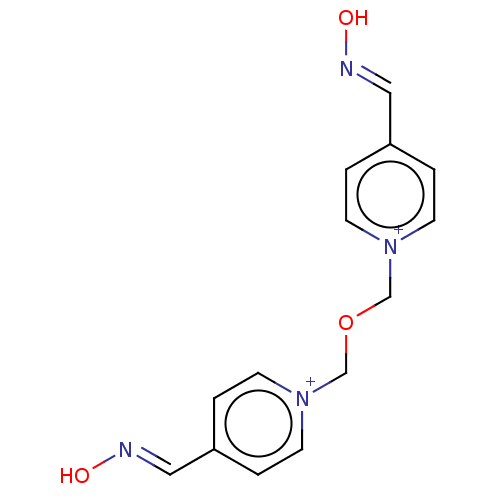 (CHEMBL139148 | Obidoxime) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of O-ethylsarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DT... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108607 (CHEMBL3597939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108605 (CHEMBL3597940) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.83E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108604 (CHEMBL3597941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108603 (CHEMBL3596215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.51E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108602 (CHEMBL3597942) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.72E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108610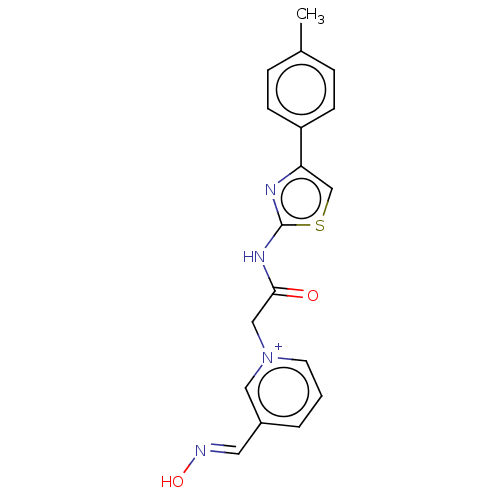 (CHEMBL3597943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108601 (CHEMBL3597944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.36E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108609 (CHEMBL3597945) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108600 (CHEMBL3597946) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108599 (CHEMBL3597947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.77E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108574 (CHEMBL3597948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108608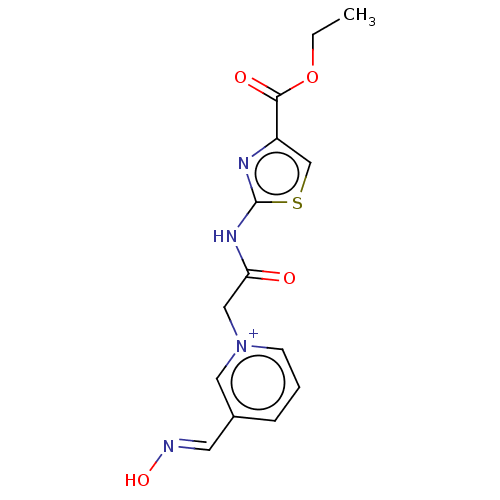 (CHEMBL3597949) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.84E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005579 (CHEMBL139148 | Obidoxime) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187213 (CHEMBL3828586) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187214 (CHEMBL3828385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187215 (CHEMBL3828381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.55E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187216 (CHEMBL3827575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187213 (CHEMBL3828586) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.67E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187214 (CHEMBL3828385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187215 (CHEMBL3828381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187216 (CHEMBL3827575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment Curated by ChEMBL | Assay Description Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... | Bioorg Med Chem 24: 4171-4176 (2016) Article DOI: 10.1016/j.bmc.2016.07.005 BindingDB Entry DOI: 10.7270/Q25D8TSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108608 (CHEMBL3597949) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.55E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE) Curated by ChEMBL | Assay Description Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ... | Bioorg Med Chem 23: 4899-910 (2015) Article DOI: 10.1016/j.bmc.2015.05.027 BindingDB Entry DOI: 10.7270/Q2GQ70J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |