 Found 201 hits with Last Name = 'adam' and Initial = 'gc'
Found 201 hits with Last Name = 'adam' and Initial = 'gc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50258579
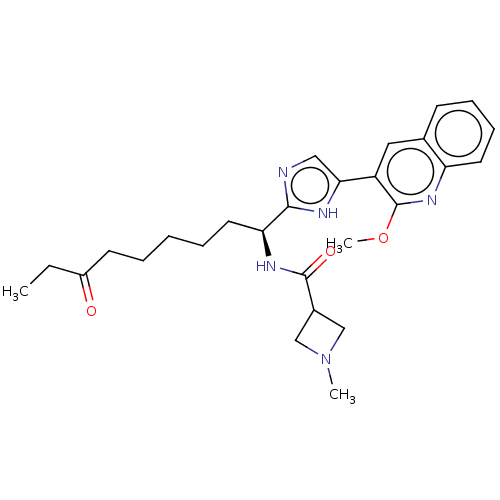
((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569630
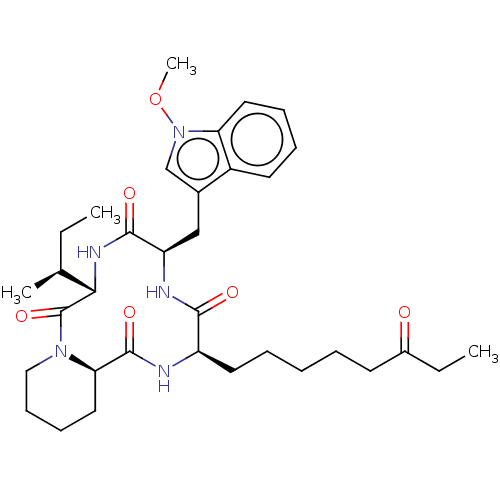
(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5290844
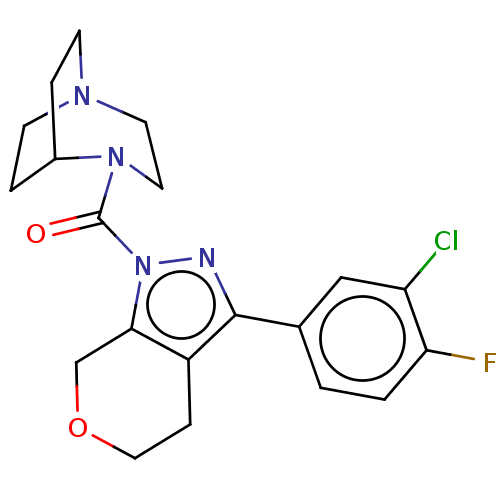
Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5275403

Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15559

((2S)-2-[4-({[(2S)-1-[(3R)-3-amino-4-(2,4,5-trifluo...)Show SMILES CC(C)[C@H](Oc1ccc(CNC(=O)[C@@H]2CCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C27H32F3N3O5/c1-15(2)25(27(36)37)38-19-7-5-16(6-8-19)14-32-26(35)23-4-3-9-33(23)24(34)12-18(31)10-17-11-21(29)22(30)13-20(17)28/h5-8,11,13,15,18,23,25H,3-4,9-10,12,14,31H2,1-2H3,(H,32,35)(H,36,37)/t18-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5291288

Show SMILES CC(C)c1c(CCP(O)(O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5277419
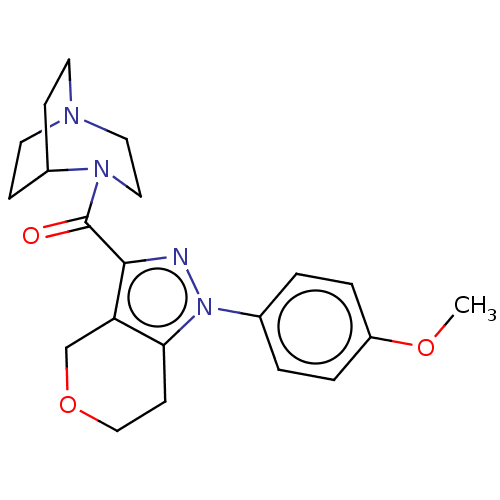
Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1CCP(O)(O)CC(=O)CC(O)=O Show InChI InChI=1S/C23H30FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-6,9-11,14,28-30H,7-8,12-13H2,1-4H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25150
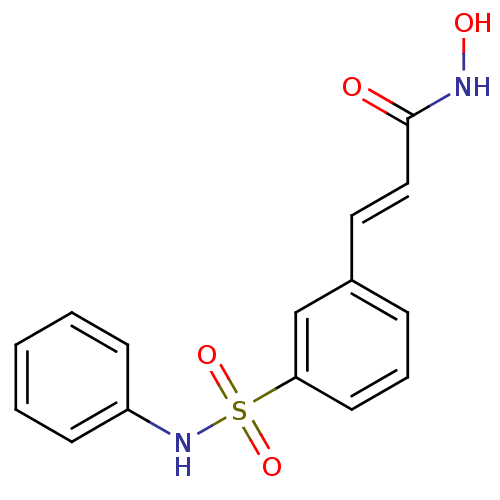
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15560
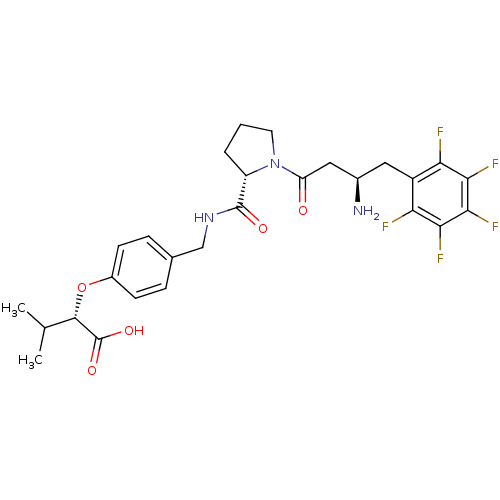
((2S)-2-[4-({[(2S)-1-[(3R)-3-amino-4-(2,3,4,5,6-pen...)Show SMILES CC(C)[C@H](Oc1ccc(CNC(=O)[C@@H]2CCCN2C(=O)C[C@H](N)Cc2c(F)c(F)c(F)c(F)c2F)cc1)C(O)=O |r| Show InChI InChI=1S/C27H30F5N3O5/c1-13(2)25(27(38)39)40-16-7-5-14(6-8-16)12-34-26(37)18-4-3-9-35(18)19(36)11-15(33)10-17-20(28)22(30)24(32)23(31)21(17)29/h5-8,13,15,18,25H,3-4,9-12,33H2,1-2H3,(H,34,37)(H,38,39)/t15-,18+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123
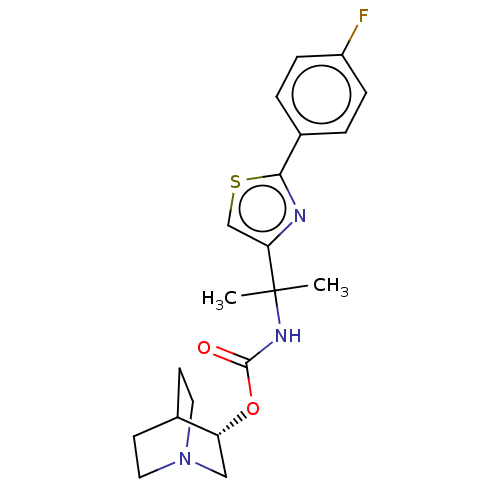
(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against bovine cathepsin D |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | CHEMBL5275403

Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | CHEMBL5272946

Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5273314

Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15561
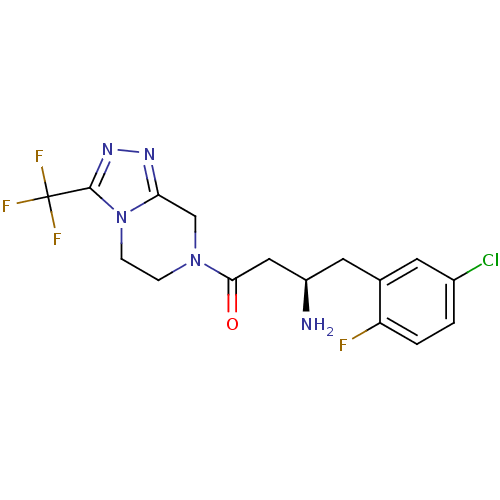
((3R)-3-amino-4-(5-chloro-2-fluorophenyl)-1-[3-(tri...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(Cl)ccc1F |r| Show InChI InChI=1S/C16H16ClF4N5O/c17-10-1-2-12(18)9(5-10)6-11(22)7-14(27)25-3-4-26-13(8-25)23-24-15(26)16(19,20)21/h1-2,5,11H,3-4,6-8,22H2/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5286066

Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5278676

Show SMILES CCc1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:5.5| Show InChI InChI=1S/C22H21FNO5P/c1-2-18-19-5-3-4-6-20(19)24(16-9-7-15(23)8-10-16)21(18)11-12-30(28,29)14-17(25)13-22(26)27/h3-12H,2,13-14H2,1H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against aspartic proteinases pepsin from porcine |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5275403

Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5288633

Show SMILES COP(=O)(C[C@@H](O)CC(O)=O)NCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H27FNO5P/c1-13-7-14(2)19(18(8-13)16-5-6-20(22)15(3)9-16)11-23-29(27,28-4)12-17(24)10-21(25)26/h5-9,17,24H,10-12H2,1-4H3,(H,23,27)(H,25,26)/t17-,29?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5286071
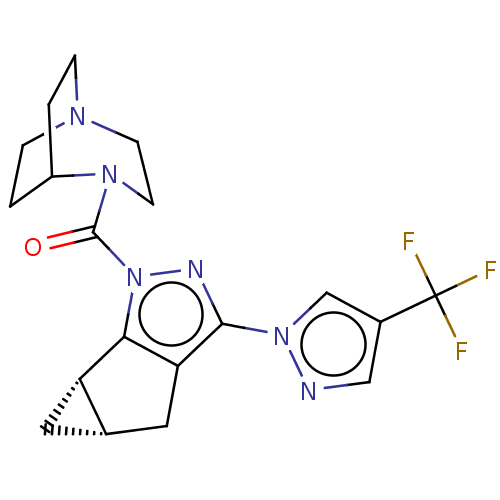
Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15562
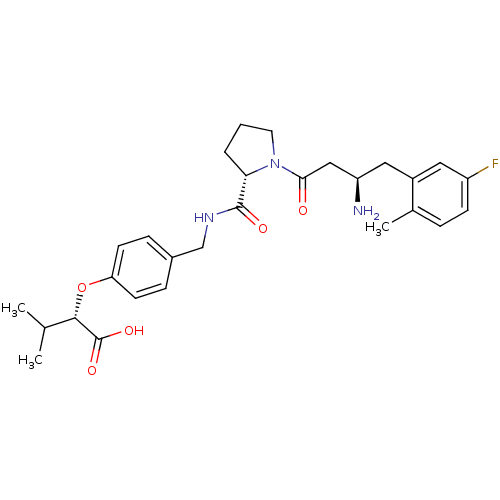
((2S)-2-[4-({[(2S)-1-[(3R)-3-amino-4-(5-fluoro-2-me...)Show SMILES CC(C)[C@H](Oc1ccc(CNC(=O)[C@@H]2CCCN2C(=O)C[C@H](N)Cc2cc(F)ccc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H36FN3O5/c1-17(2)26(28(35)36)37-23-10-7-19(8-11-23)16-31-27(34)24-5-4-12-32(24)25(33)15-22(30)14-20-13-21(29)9-6-18(20)3/h6-11,13,17,22,24,26H,4-5,12,14-16,30H2,1-3H3,(H,31,34)(H,35,36)/t22-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5272946

Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5278405
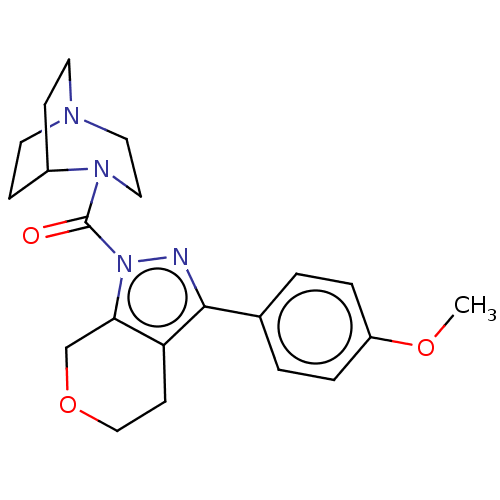
Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569640

(CHEMBL4861270)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5278640

Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15563
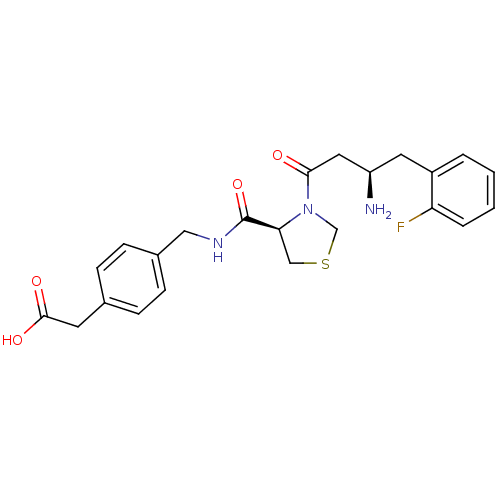
(2-[4-({[(4R)-3-[(3R)-3-amino-4-(2-fluorophenyl)but...)Show SMILES N[C@@H](CC(=O)N1CSC[C@H]1C(=O)NCc1ccc(CC(O)=O)cc1)Cc1ccccc1F |r| Show InChI InChI=1S/C23H26FN3O4S/c24-19-4-2-1-3-17(19)10-18(25)11-21(28)27-14-32-13-20(27)23(31)26-12-16-7-5-15(6-8-16)9-22(29)30/h1-8,18,20H,9-14,25H2,(H,26,31)(H,29,30)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123

(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569641

(CHEMBL4872186)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569640

(CHEMBL4861270)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5273405

Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1COP(O)(=O)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C22H28FO6P/c1-13(2)18-7-14(3)8-19(16-5-6-21(23)15(4)9-16)20(18)11-29-30(27,28)12-17(24)10-22(25)26/h5-9,13,17,24H,10-12H2,1-4H3,(H,25,26)(H,27,28)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569640

(CHEMBL4861270)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15564
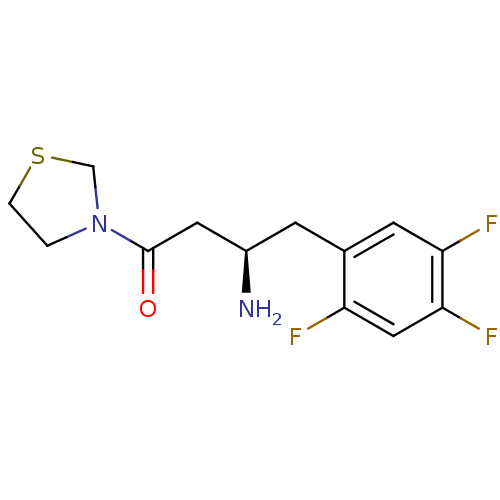
((3R)-3-amino-1-(1,3-thiazolidin-3-yl)-4-(2,4,5-tri...)Show InChI InChI=1S/C13H15F3N2OS/c14-10-6-12(16)11(15)4-8(10)3-9(17)5-13(19)18-1-2-20-7-18/h4,6,9H,1-3,5,7,17H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of class 1 HDAC in human Jurkat 2C4 model of HIV latency assessed as reactivation of HIV latency incubated for 18 to 24 hrs in presence of... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569641

(CHEMBL4872186)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569633
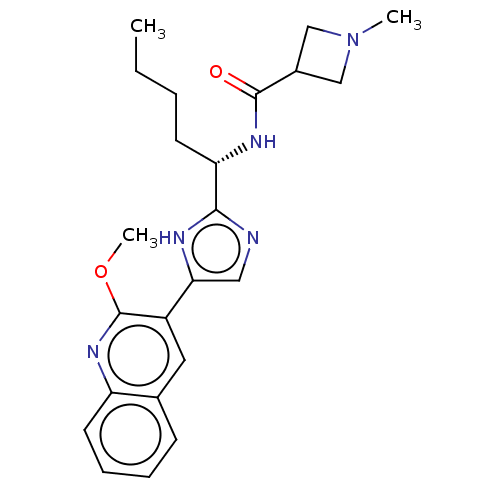
(CHEMBL4851511)Show SMILES CCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15565

(DPP-4 ligand 7 | methyl 2-[4-({[(2S)-1-[(3R)-3-ami...)Show SMILES COC(=O)Cc1ccc(CNC(=O)[C@@H]2CCCN2C(=O)C[C@H](N)Cc2ccccc2F)cc1 |r| Show InChI InChI=1S/C25H30FN3O4/c1-33-24(31)13-17-8-10-18(11-9-17)16-28-25(32)22-7-4-12-29(22)23(30)15-20(27)14-19-5-2-3-6-21(19)26/h2-3,5-6,8-11,20,22H,4,7,12-16,27H2,1H3,(H,28,32)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 2404-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.040
BindingDB Entry DOI: 10.7270/Q2M906WF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5270537

Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of class 1 HDAC in human Jurkat 2C4 model of HIV latency assessed as reactivation of HIV latency incubated for 18 to 24 hrs in presence of... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569641

(CHEMBL4872186)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | CHEMBL5272946

Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data