 Found 1422 hits with Last Name = 'adams' and Initial = 'c'
Found 1422 hits with Last Name = 'adams' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338686

((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C22H23ClN4O3S/c1-24-19-7-2-13-10-14(3-5-16(13)19)27-9-8-20(22(27)28)26-31(29,30)15-4-6-17-18(23)12-25-21(17)11-15/h3-6,10-12,19-20,24-26H,2,7-9H2,1H3/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339708

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-17-4-2-13(28-17)7-10-29(26,27)23-15-6-9-24(19(15)25)16-3-1-12-11-22-8-5-14(12)18(16)21/h1-4,7,10,15,22-23H,5-6,8-9,11H2/b10-7+/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339718
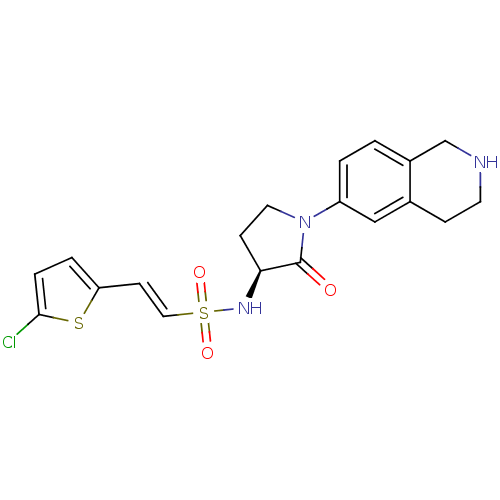
((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-14-12-21-8-5-13(14)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339716

((S)-6-chloro-N-(1-(6-fluoro-2,3,4,5-tetrahydro-1H-...)Show SMILES Fc1c2CCCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-21-9-11-29(24(21)30)22-8-5-17-14-27-10-1-2-20(17)23(22)26/h3-8,12-13,21,27-28H,1-2,9-11,14H2/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339714
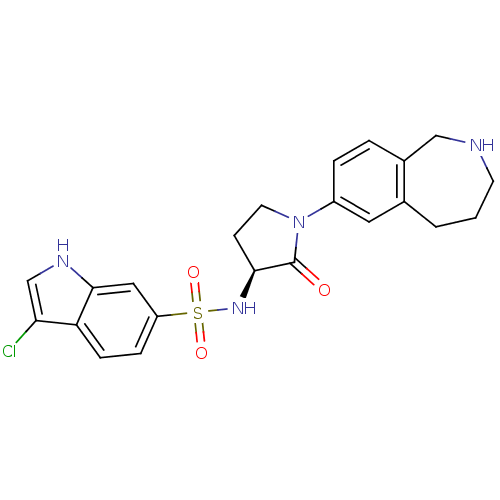
((S)-3-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C22H23ClN4O3S/c23-19-13-25-21-11-17(5-6-18(19)21)31(29,30)26-20-7-9-27(22(20)28)16-4-3-15-12-24-8-1-2-14(15)10-16/h3-6,10-11,13,20,24-26H,1-2,7-9,12H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339713
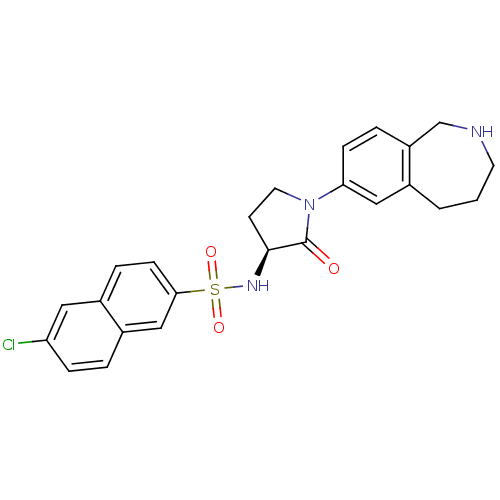
((S)-6-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C24H24ClN3O3S/c25-20-6-3-18-14-22(8-5-17(18)12-20)32(30,31)27-23-9-11-28(24(23)29)21-7-4-19-15-26-10-1-2-16(19)13-21/h3-8,12-14,23,26-27H,1-2,9-11,15H2/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338689

((R/S)-3-chloro-N-((3S)-1-(1-(dimethylamino)-2,3-di...)Show SMILES CN(C)C1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C23H25ClN4O3S/c1-27(2)22-8-3-14-11-15(4-6-17(14)22)28-10-9-20(23(28)29)26-32(30,31)16-5-7-18-19(24)13-25-21(18)12-16/h4-7,11-13,20,22,25-26H,3,8-10H2,1-2H3/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339717

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(2-methyl-1,2,3...)Show SMILES CN1CCc2cc(ccc2C1)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H22ClN3O3S2/c1-23-9-6-14-12-16(3-2-15(14)13-23)24-10-7-18(20(24)25)22-29(26,27)11-8-17-4-5-19(21)28-17/h2-5,8,11-12,18,22H,6-7,9-10,13H2,1H3/b11-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339711

((S)-3-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C21H20ClFN4O3S/c22-16-11-25-19-9-14(1-2-15(16)19)31(29,30)26-18-4-6-27(21(18)28)20-8-12-3-5-24-10-13(12)7-17(20)23/h1-2,7-9,11,18,24-26H,3-6,10H2/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339712

((S,E)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(2,3,4,...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCCc3c2)s1 |r| Show InChI InChI=1S/C20H22ClN3O3S2/c21-19-6-5-17(28-19)8-11-29(26,27)23-18-7-10-24(20(18)25)16-4-3-15-13-22-9-1-2-14(15)12-16/h3-6,8,11-12,18,22-23H,1-2,7,9-10,13H2/b11-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339719
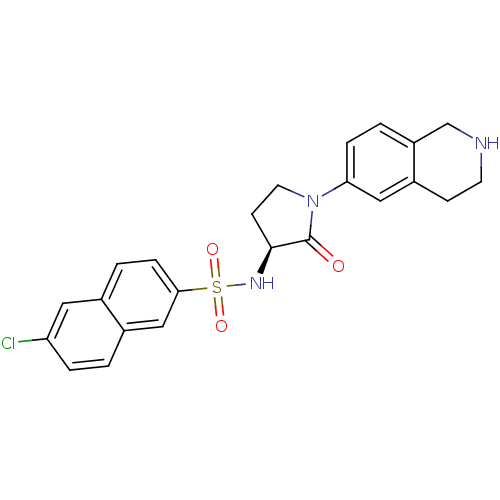
((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-19-4-1-16-13-21(6-3-15(16)11-19)31(29,30)26-22-8-10-27(23(22)28)20-5-2-18-14-25-9-7-17(18)12-20/h1-6,11-13,22,25-26H,7-10,14H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339720
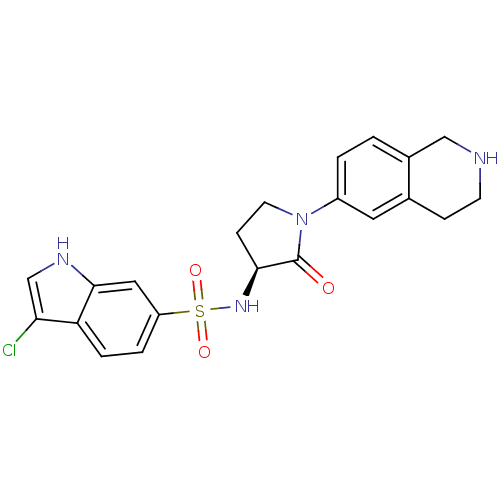
((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C21H21ClN4O3S/c22-18-12-24-20-10-16(3-4-17(18)20)30(28,29)25-19-6-8-26(21(19)27)15-2-1-14-11-23-7-5-13(14)9-15/h1-4,9-10,12,19,23-25H,5-8,11H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339715
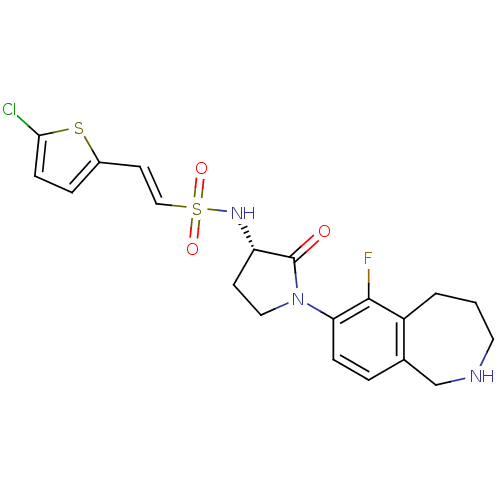
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(6-fluoro-2,3,4...)Show SMILES Fc1c2CCCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-18-6-4-14(29-18)8-11-30(27,28)24-16-7-10-25(20(16)26)17-5-3-13-12-23-9-1-2-15(13)19(17)22/h3-6,8,11,16,23-24H,1-2,7,9-10,12H2/b11-8+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339706

((S)-6-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H21ClFN3O3S/c24-17-4-1-15-12-18(5-2-14(15)11-17)32(30,31)27-20-8-10-28(23(20)29)21-6-3-16-13-26-9-7-19(16)22(21)25/h1-6,11-12,20,26-27H,7-10,13H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
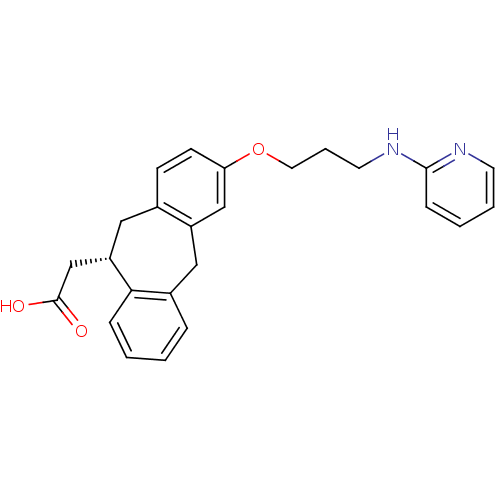
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133
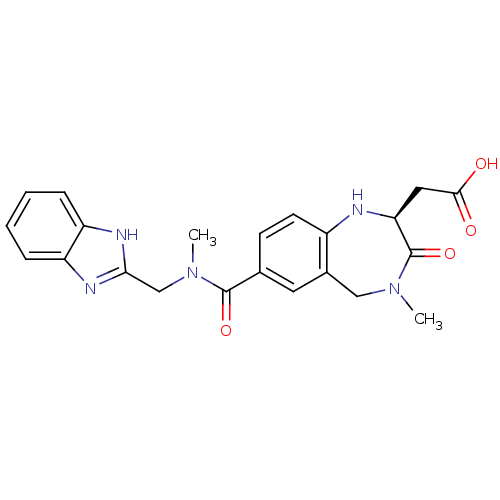
(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of HEK 293 cell adhesion to vitronectin by alpha V beta 3 |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338691

(6-CHLORO-N-[(3S)-1-[(1S)-1-DIMETHYLAMINO-2,3-DIHYD...)Show SMILES CN(C)[C@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306134
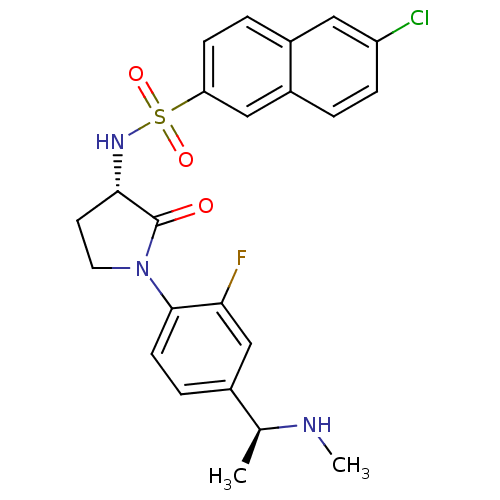
(6-chloro-N-((S)-1-(2-fluoro-4-((S)-1-(methylamino)...)Show SMILES CN[C@@H](C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H23ClFN3O3S/c1-14(26-2)15-5-8-22(20(25)13-15)28-10-9-21(23(28)29)27-32(30,31)19-7-4-16-11-18(24)6-3-17(16)12-19/h3-8,11-14,21,26-27H,9-10H2,1-2H3/t14-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339710
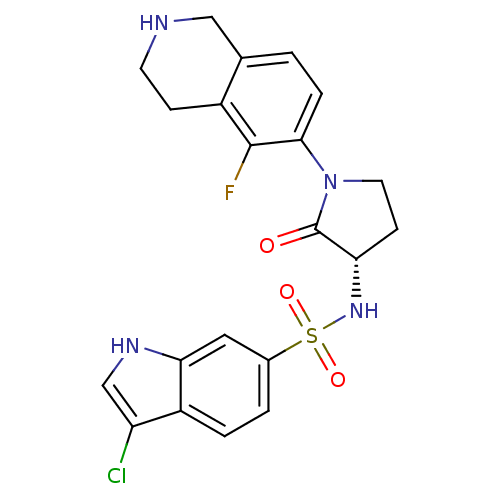
((S)-3-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C21H20ClFN4O3S/c22-16-11-25-18-9-13(2-3-15(16)18)31(29,30)26-17-6-8-27(21(17)28)19-4-1-12-10-24-7-5-14(12)20(19)23/h1-4,9,11,17,24-26H,5-8,10H2/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339709
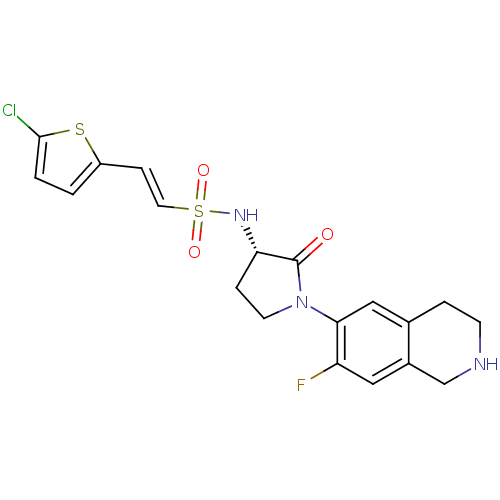
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(7-fluoro-1,2,3...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-18-2-1-14(28-18)5-8-29(26,27)23-16-4-7-24(19(16)25)17-10-12-3-6-22-11-13(12)9-15(17)21/h1-2,5,8-10,16,22-23H,3-4,6-7,11H2/b8-5+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306134

(6-chloro-N-((S)-1-(2-fluoro-4-((S)-1-(methylamino)...)Show SMILES CN[C@@H](C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H23ClFN3O3S/c1-14(26-2)15-5-8-22(20(25)13-15)28-10-9-21(23(28)29)27-32(30,31)19-7-4-16-11-18(24)6-3-17(16)12-19/h3-8,11-14,21,26-27H,9-10H2,1-2H3/t14-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338677

(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2S)-2-PYRROLIDINYL]P...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338682
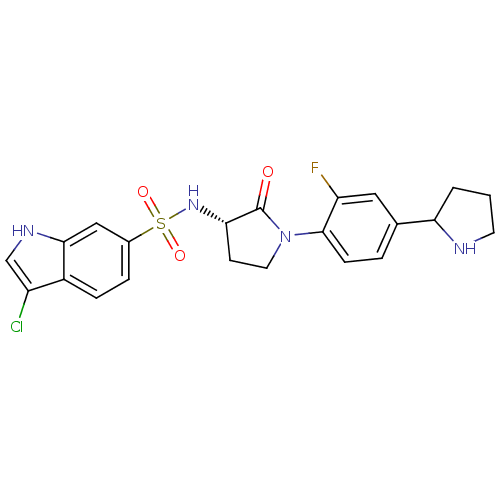
((R/S)-3-chloro-N-((3S)-1-(2-fluoro-4-(pyrrolidin-2...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O)C1CCCN1 |r| Show InChI InChI=1S/C22H22ClFN4O3S/c23-16-12-26-20-11-14(4-5-15(16)20)32(30,31)27-19-7-9-28(22(19)29)21-6-3-13(10-17(21)24)18-2-1-8-25-18/h3-6,10-12,18-19,25-27H,1-2,7-9H2/t18?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338678
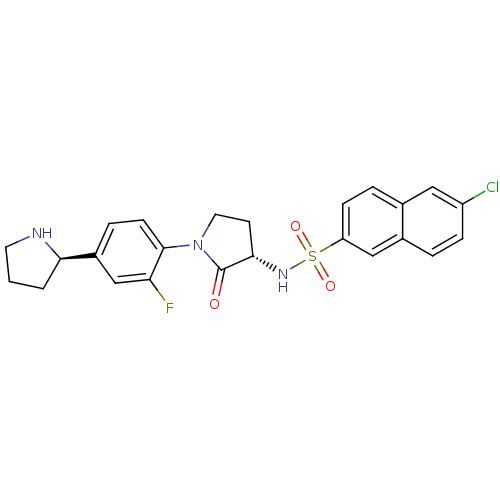
(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2R)-2--PYRROLIDINYL]...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338690

(6-CHLORO-N-{(3S)-1-[(1S)-1-(DIMETHYLAMINO)-2,3-DIH...)Show SMILES CN(C)[C@@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338680
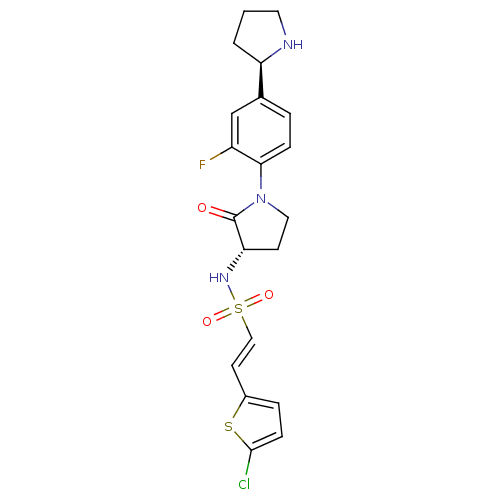
((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339707
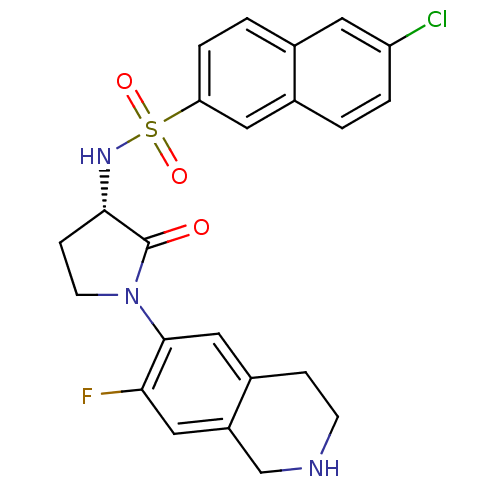
((S)-6-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H21ClFN3O3S/c24-18-3-1-15-10-19(4-2-14(15)9-18)32(30,31)27-21-6-8-28(23(21)29)22-12-16-5-7-26-13-17(16)11-20(22)25/h1-4,9-12,21,26-27H,5-8,13H2/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17643
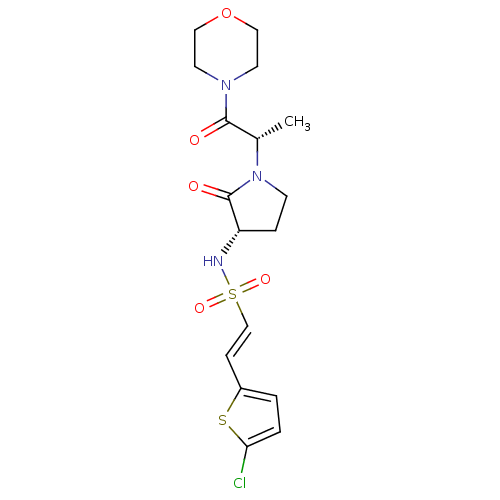
((E)-2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(m...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C17H22ClN3O5S2/c1-12(16(22)20-7-9-26-10-8-20)21-6-4-14(17(21)23)19-28(24,25)11-5-13-2-3-15(18)27-13/h2-3,5,11-12,14,19H,4,6-10H2,1H3/b11-5+/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338688
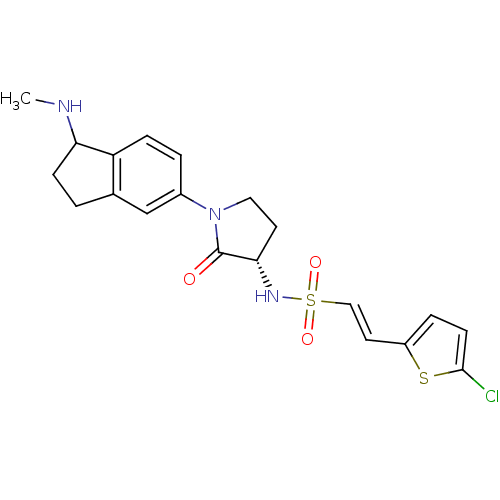
((R/S)-(E)-2-(5-chlorothiophen-2-yl)-N-((3S)-1-(1-(...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H22ClN3O3S2/c1-22-17-6-2-13-12-14(3-5-16(13)17)24-10-8-18(20(24)25)23-29(26,27)11-9-15-4-7-19(21)28-15/h3-5,7,9,11-12,17-18,22-23H,2,6,8,10H2,1H3/b11-9+/t17?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50310456
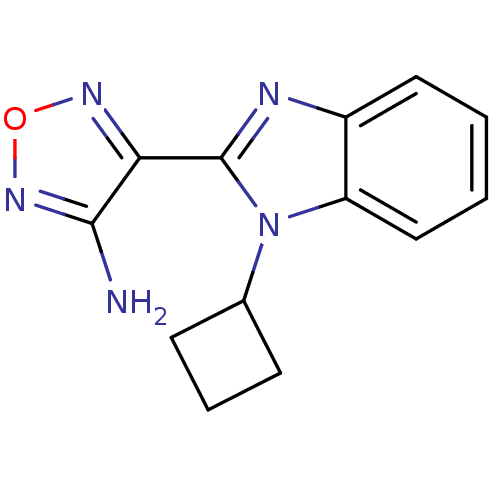
(4-(1-cyclobutyl-1H-benzo[d]imidazol-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C13H13N5O/c14-12-11(16-19-17-12)13-15-9-6-1-2-7-10(9)18(13)8-4-3-5-8/h1-2,6-8H,3-5H2,(H2,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K assessed as decrease in NADH absorbance at 340 nm in the presence of |
Bioorg Med Chem Lett 19: 5191-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.022
BindingDB Entry DOI: 10.7270/Q2KP8287 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of HEK 293 cell adhesion to vitronectin by alpha V beta 3 |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338692

((R/S)-(E)-2-(5-chlorothiophen-2-yl)-N-((3S)-1-(1-(...)Show SMILES CN(C)C1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C21H24ClN3O3S2/c1-24(2)19-7-3-14-13-15(4-6-17(14)19)25-11-9-18(21(25)26)23-30(27,28)12-10-16-5-8-20(22)29-16/h4-6,8,10,12-13,18-19,23H,3,7,9,11H2,1-2H3/b12-10+/t18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338677

(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2S)-2-PYRROLIDINYL]P...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315675
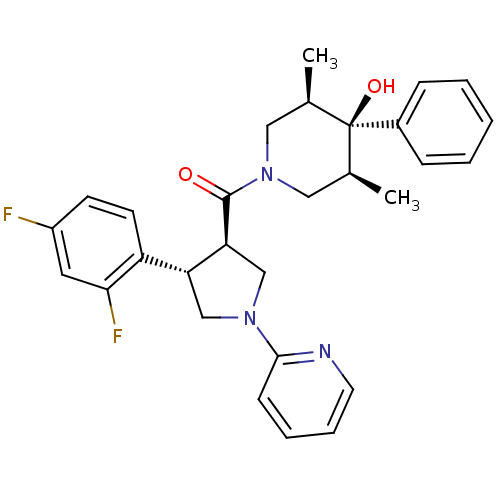
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-2-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccccn1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-8-4-3-5-9-21)28(35)25-18-33(27-10-6-7-13-32-27)17-24(25)23-12-11-22(30)14-26(23)31/h3-14,19-20,24-25,36H,15-18H2,1-2H3/t19-,20+,24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338687

((R/S)-6-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H24ClN3O3S/c1-26-22-9-4-17-13-19(6-8-21(17)22)28-11-10-23(24(28)29)27-32(30,31)20-7-3-15-12-18(25)5-2-16(15)14-20/h2-3,5-8,12-14,22-23,26-27H,4,9-11H2,1H3/t22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315688
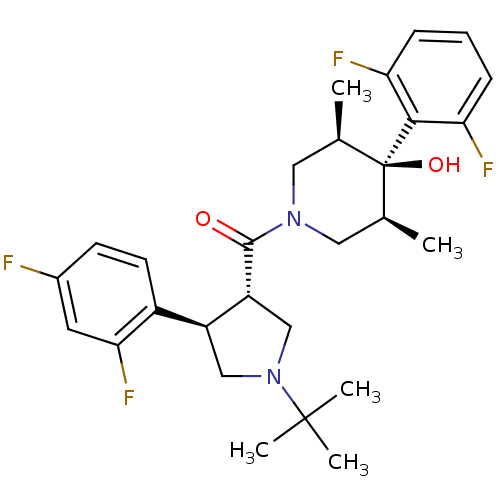
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1c(F)cccc1F)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F4N2O2/c1-16-12-33(13-17(2)28(16,36)25-22(30)7-6-8-23(25)31)26(35)21-15-34(27(3,4)5)14-20(21)19-10-9-18(29)11-24(19)32/h6-11,16-17,20-21,36H,12-15H2,1-5H3/t16-,17+,20-,21+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338679

((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338685

((R/S)-N-((3S)-1-(1-amino-2,3-dihydro-1H-inden-5-yl...)Show SMILES NC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-17-4-1-15-13-19(6-2-14(15)11-17)31(29,30)26-22-9-10-27(23(22)28)18-5-7-20-16(12-18)3-8-21(20)25/h1-2,4-7,11-13,21-22,26H,3,8-10,25H2/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315674
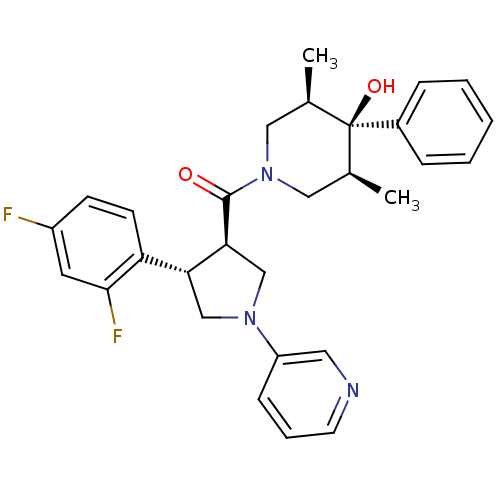
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315676
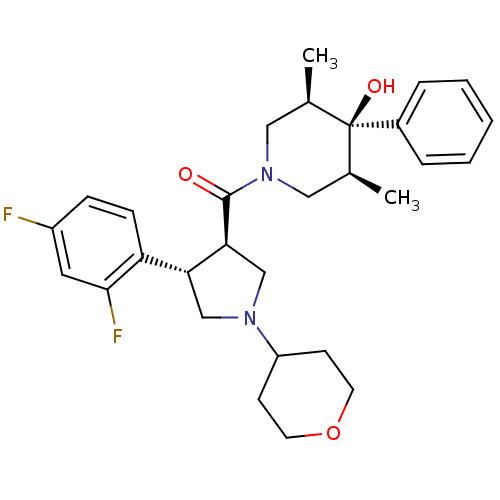
(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315686
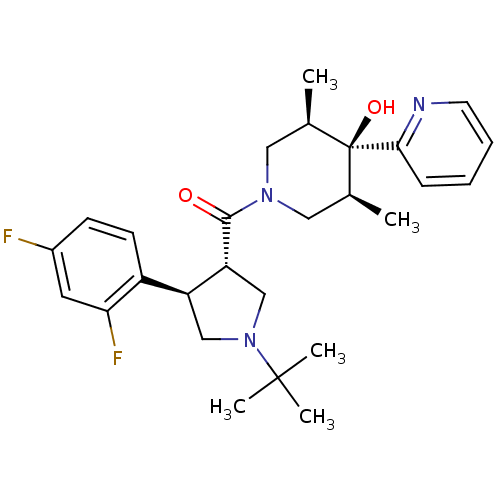
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C27H35F2N3O2/c1-17-13-31(14-18(2)27(17,34)24-8-6-7-11-30-24)25(33)22-16-32(26(3,4)5)15-21(22)20-10-9-19(28)12-23(20)29/h6-12,17-18,21-22,34H,13-16H2,1-5H3/t17-,18+,21-,22+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338679

((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338683

((R/S)-6-chloro-N-((3S)-1-(2-fluoro-4-(1-methylpyrr...)Show SMILES CN1CCCC1c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H25ClFN3O3S/c1-29-11-2-3-23(29)18-6-9-24(21(27)15-18)30-12-10-22(25(30)31)28-34(32,33)20-8-5-16-13-19(26)7-4-17(16)14-20/h4-9,13-15,22-23,28H,2-3,10-12H2,1H3/t22-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315680
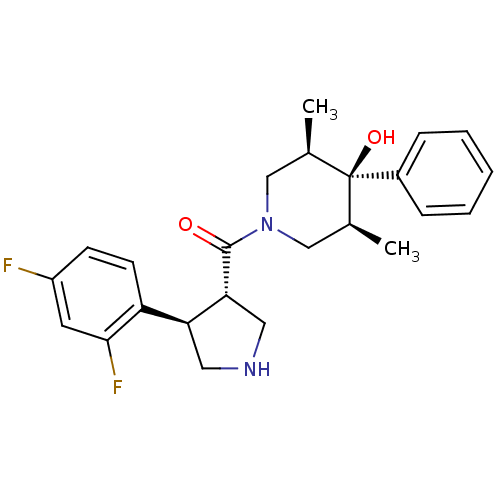
(((3S,4R)-4-(2,4-difluorophenyl)pyrrolidin-3-yl)((3...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F |r| Show InChI InChI=1S/C24H28F2N2O2/c1-15-13-28(14-16(2)24(15,30)17-6-4-3-5-7-17)23(29)21-12-27-11-20(21)19-9-8-18(25)10-22(19)26/h3-10,15-16,20-21,27,30H,11-14H2,1-2H3/t15-,16+,20-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315681
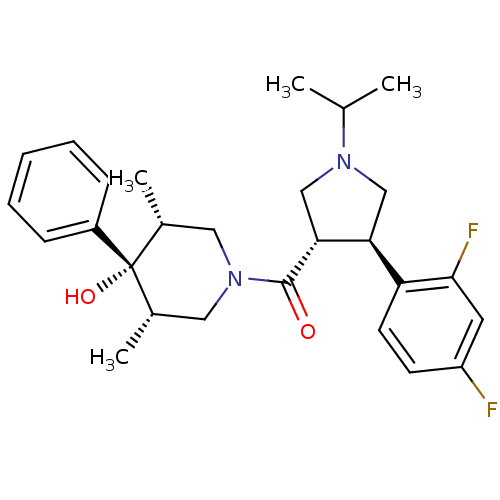
(((3S,4R)-4-(2,4-difluorophenyl)-1-isopropylpyrroli...)Show SMILES CC(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1C[C@H](C)[C@](O)([C@H](C)C1)c1ccccc1 |r| Show InChI InChI=1S/C27H34F2N2O2/c1-17(2)30-15-23(22-11-10-21(28)12-25(22)29)24(16-30)26(32)31-13-18(3)27(33,19(4)14-31)20-8-6-5-7-9-20/h5-12,17-19,23-24,33H,13-16H2,1-4H3/t18-,19+,23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
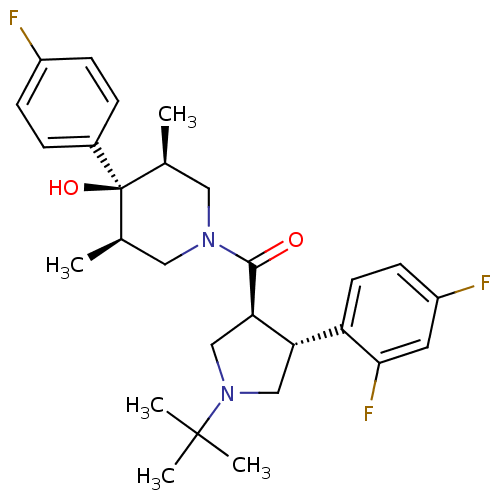
(Homo sapiens (Human)) | BDBM50378743
(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
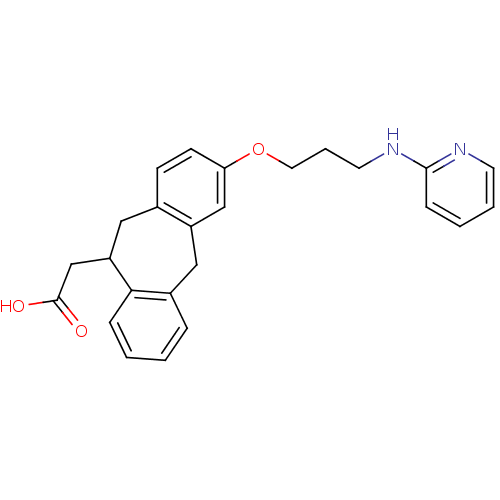
(Homo sapiens (Human)) | BDBM50078713
(CHEMBL416493 | {3-[3-(Pyridin-2-ylamino)-propoxy]-...)Show SMILES OC(=O)CC1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339721

((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CCNCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-13-5-8-21-12-14(13)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338690

(6-CHLORO-N-{(3S)-1-[(1S)-1-(DIMETHYLAMINO)-2,3-DIH...)Show SMILES CN(C)[C@@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data