

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora B ATP binding site by rapid dilution method | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Aurora A | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10323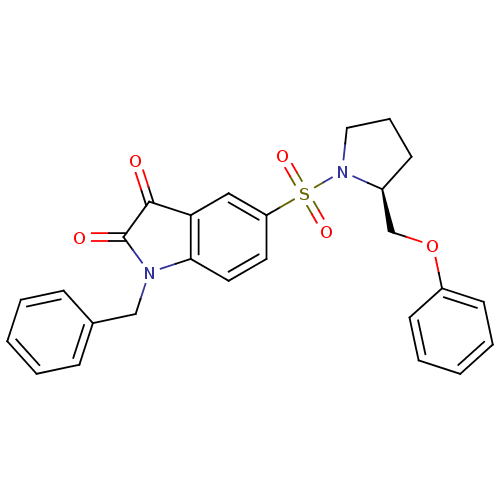 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora C ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406621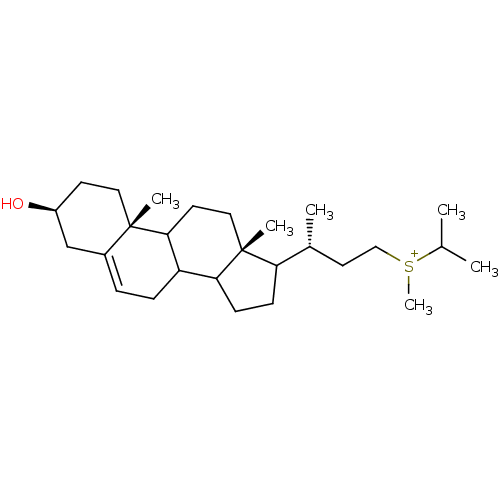 (CHEMBL9820) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora C ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406617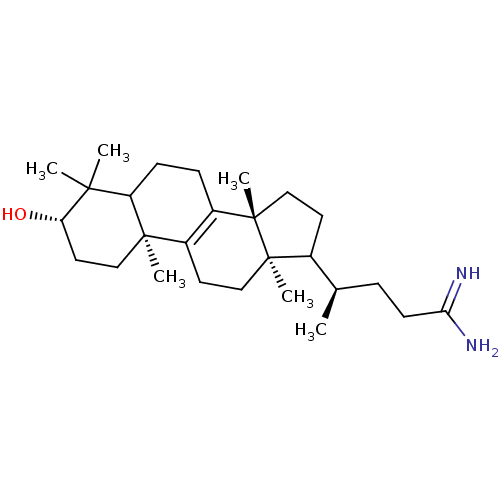 (CHEMBL276388) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406618 (CHEMBL9875) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10318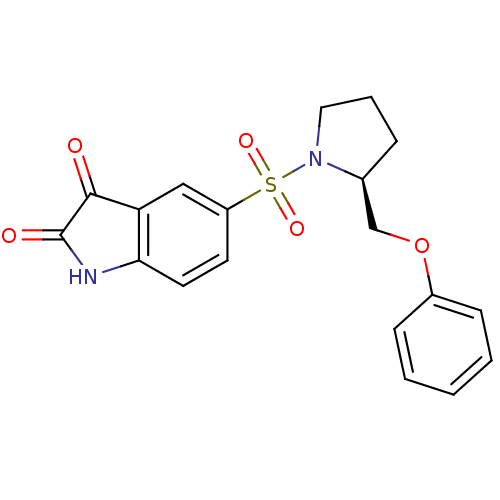 ((S)-5-{1-[2-(Phenoxymethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10315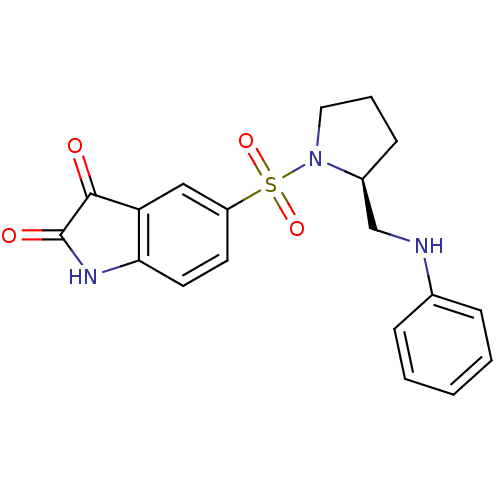 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406620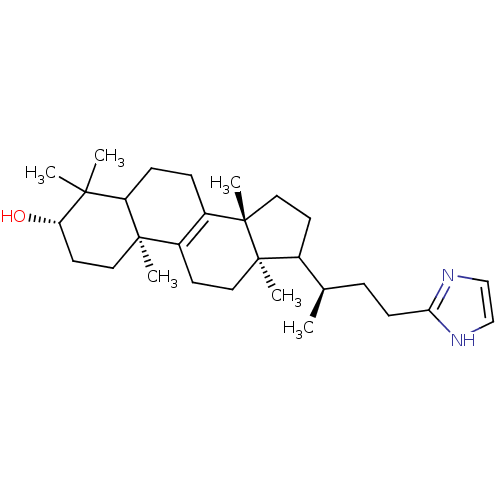 (CHEMBL9864) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10318 ((S)-5-{1-[2-(Phenoxymethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10315 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406616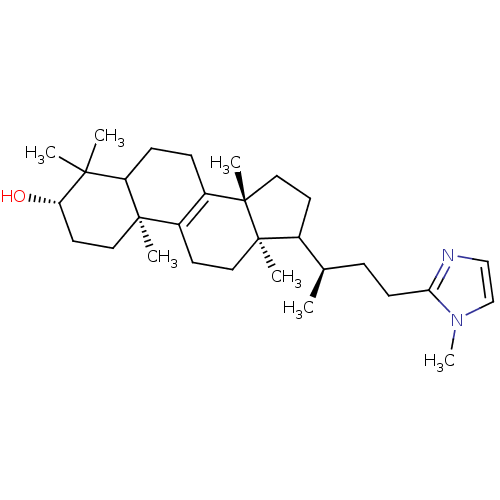 (CHEMBL268041) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10305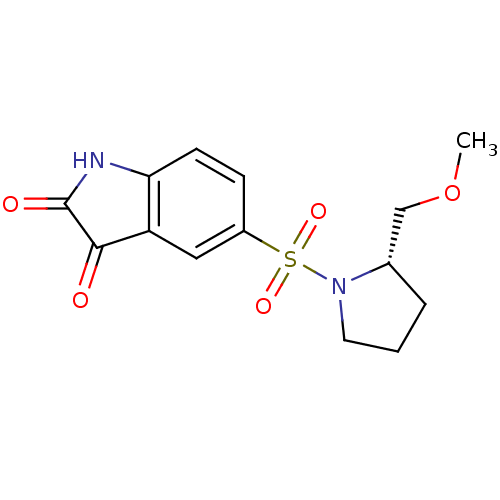 ((S)-5-[1-(2-Methoxymethyl)pyrrolidinylsulfonyl]isa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | -41.9 | 120 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10298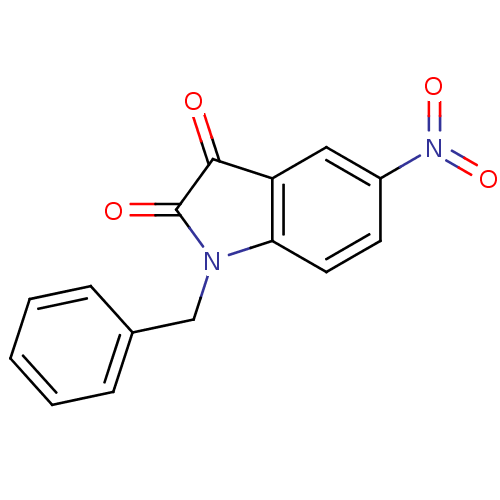 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | -40.0 | 250 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10298 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10305 ((S)-5-[1-(2-Methoxymethyl)pyrrolidinylsulfonyl]isa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10297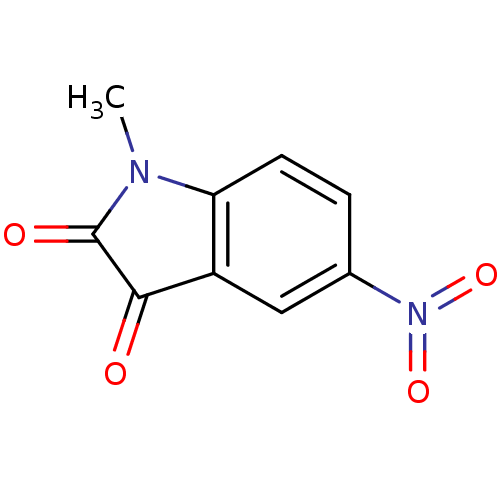 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora A ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Aurora A | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10297 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | -36.6 | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10315 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10298 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10305 ((S)-5-[1-(2-Methoxymethyl)pyrrolidinylsulfonyl]isa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10318 ((S)-5-{1-[2-(Phenoxymethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10309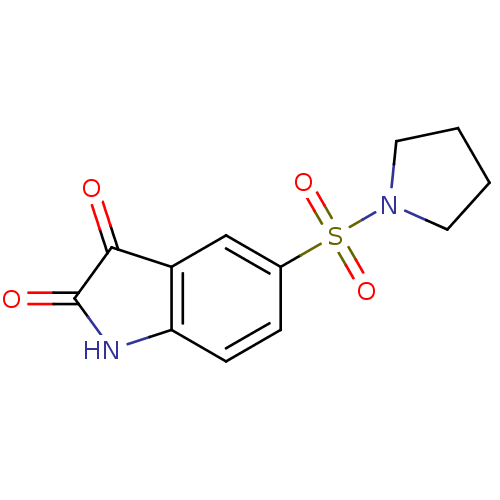 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | -34.0 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10297 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406619 (CHEMBL9808) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-4 (Homo sapiens (Human)) | BDBM10297 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10318 ((S)-5-{1-[2-(Phenoxymethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10298 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-4 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-4 (Homo sapiens (Human)) | BDBM10298 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10315 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10297 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10315 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10316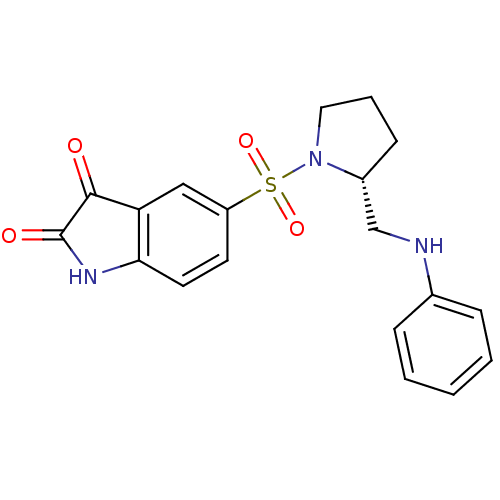 ((R)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10316 ((R)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10316 ((R)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 582 total ) | Next | Last >> |