

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120499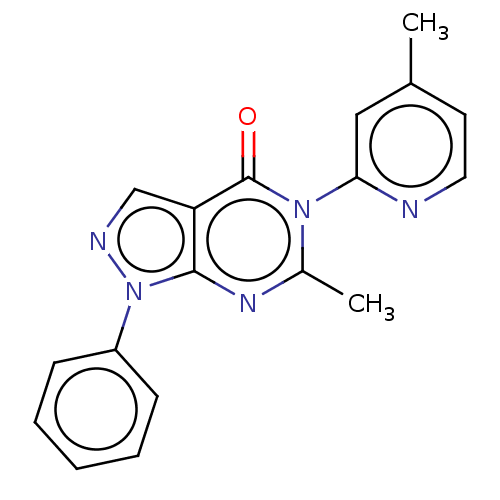 (CHEMBL3618038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120495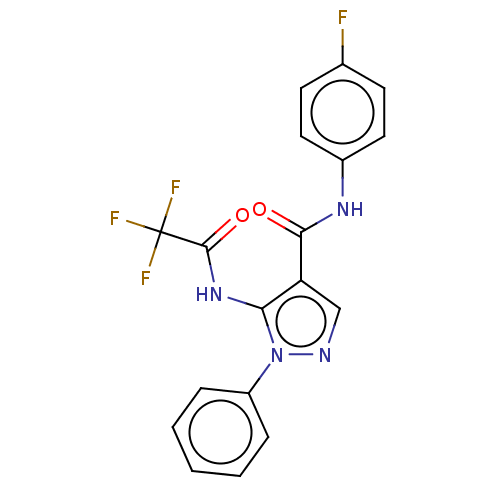 (CHEMBL3618140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120506 (CHEMBL3618032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120497 (CHEMBL3618134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120501 (CHEMBL3618036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120498 (CHEMBL3618039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120500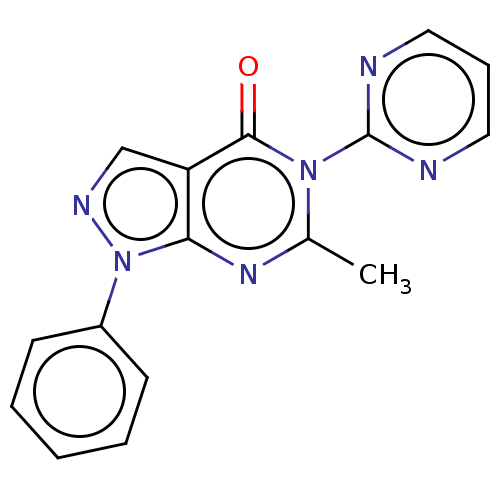 (CHEMBL3618037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50120496 (CHEMBL3618137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research-Ahmedabad Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP-4 using Gly-pro-p-nitroanilide as substrate assessed as formation of p-nitroaniline after 30 mins by colorimetric... | Bioorg Med Chem Lett 25: 4428-33 (2015) Article DOI: 10.1016/j.bmcl.2015.09.015 BindingDB Entry DOI: 10.7270/Q2XP76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||