

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033881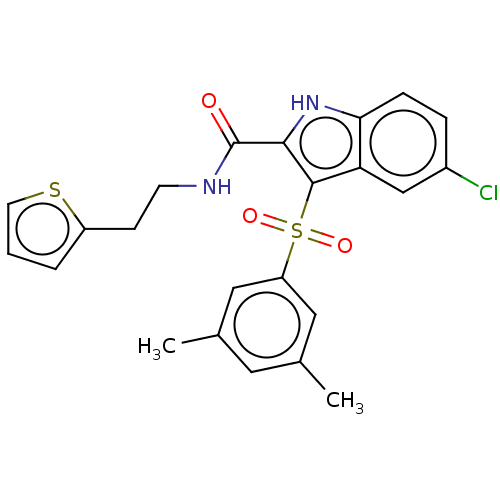 (CHEMBL3358173) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863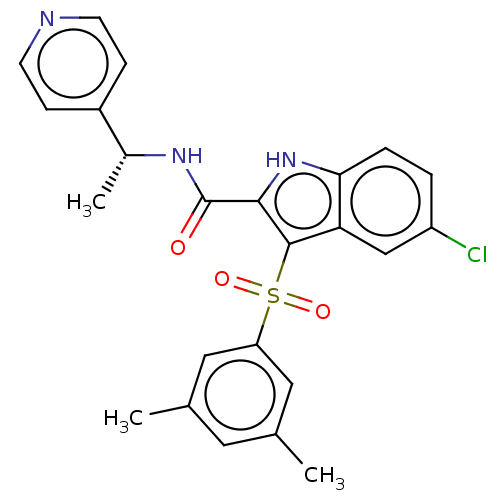 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033878 (CHEMBL3358170) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033865 (CHEMBL3358161) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase Y181I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033878 (CHEMBL3358170) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033883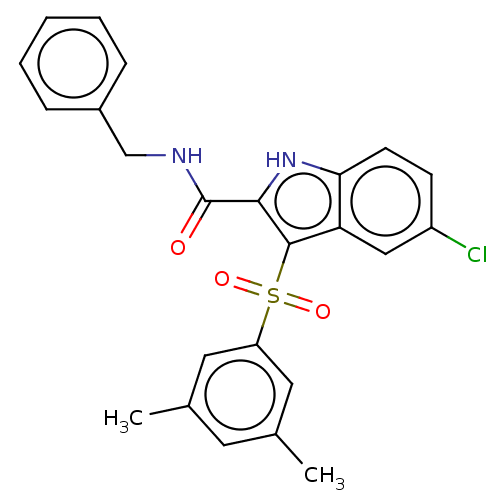 (CHEMBL1766221) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033877 (CHEMBL3358169) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033864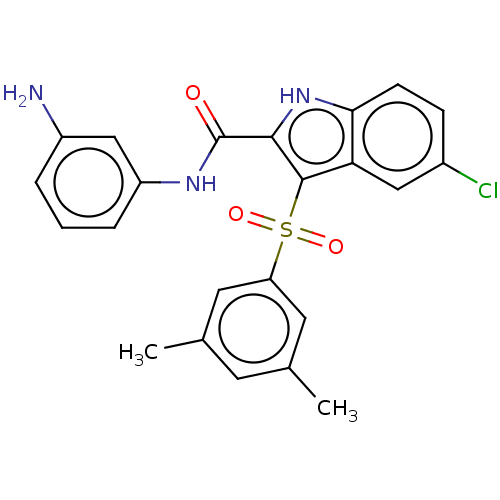 (CHEMBL3358160) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033875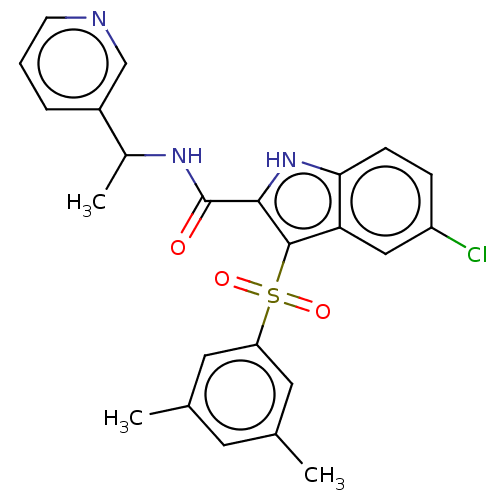 (CHEMBL3358167) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033862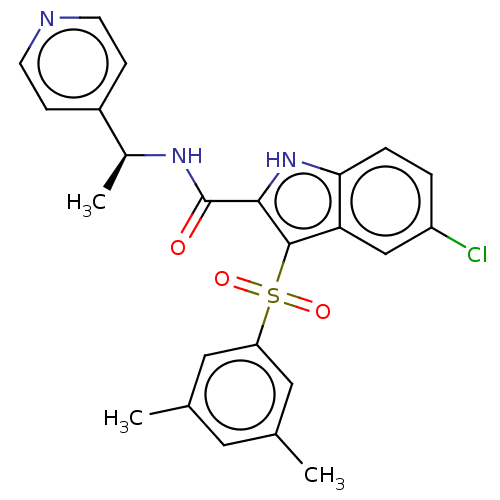 (CHEMBL3358175) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033883 (CHEMBL1766221) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033880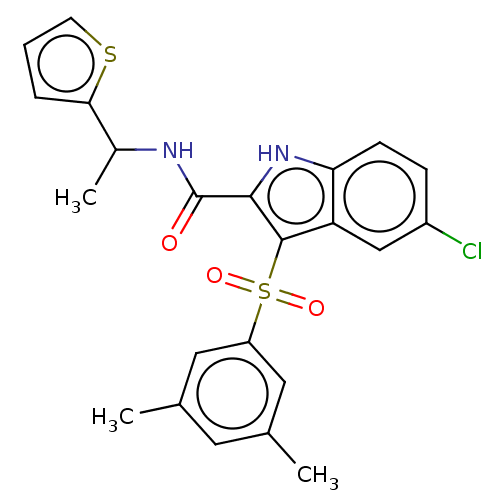 (CHEMBL3358172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033884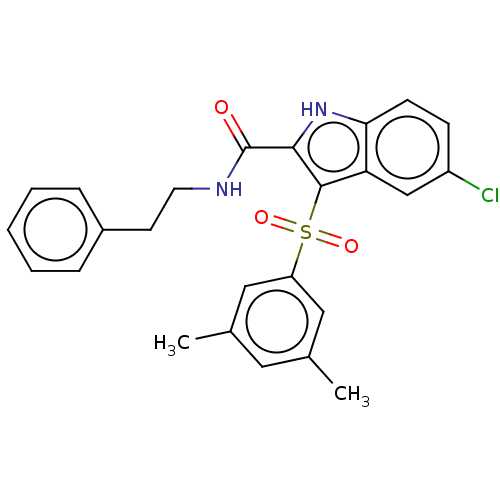 (CHEMBL1766233) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231998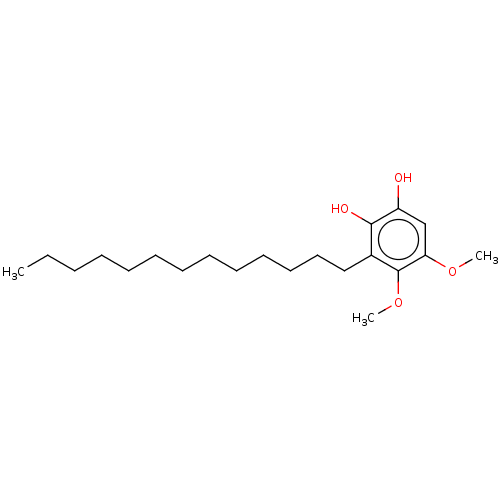 (CHEMBL4085835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078888 (CHEMBL3416359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033879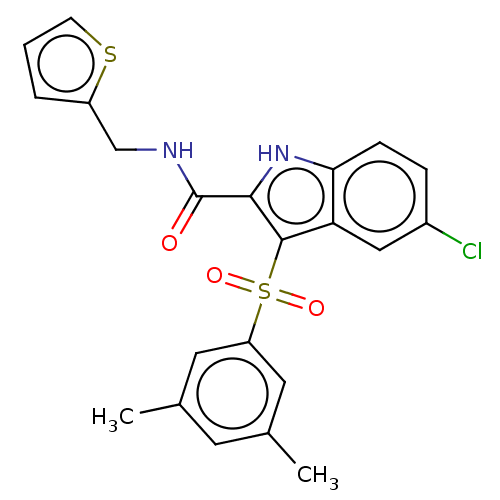 (CHEMBL3358171) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033861 (CHEMBL3358165) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231991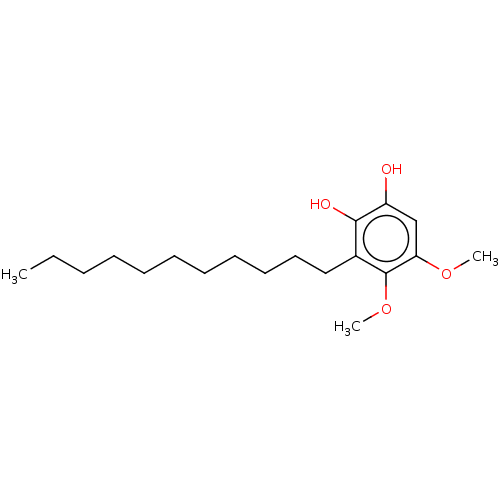 (CHEMBL4093584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033866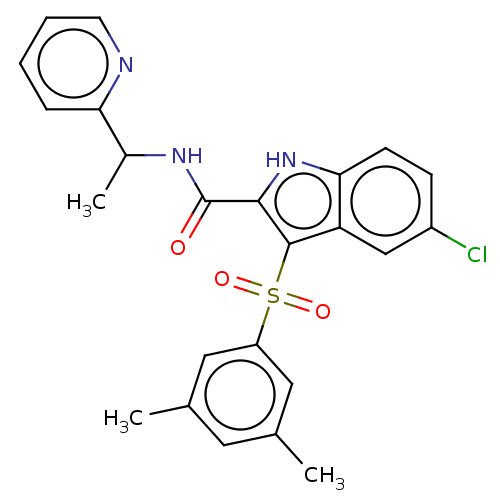 (CHEMBL3358166) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033885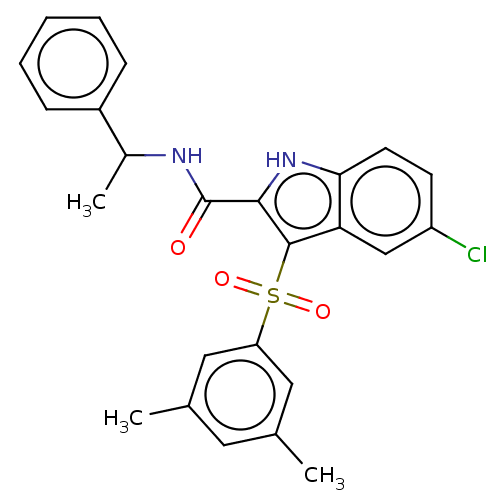 (CHEMBL3263468) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033861 (CHEMBL3358165) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033861 (CHEMBL3358165) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033862 (CHEMBL3358175) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078890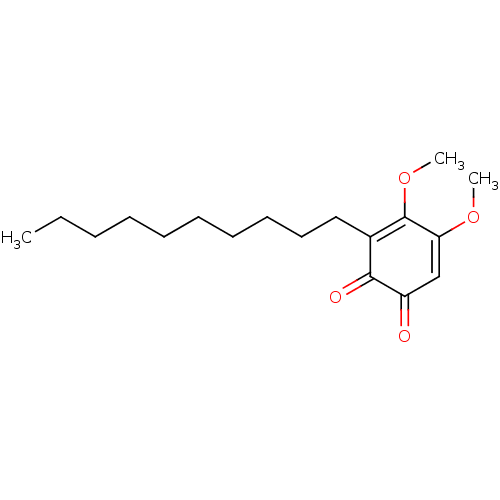 (CHEMBL3416357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078886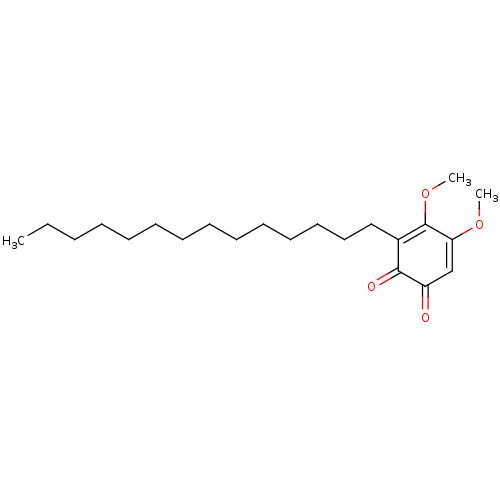 (CHEMBL3416361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033881 (CHEMBL3358173) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033865 (CHEMBL3358161) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033882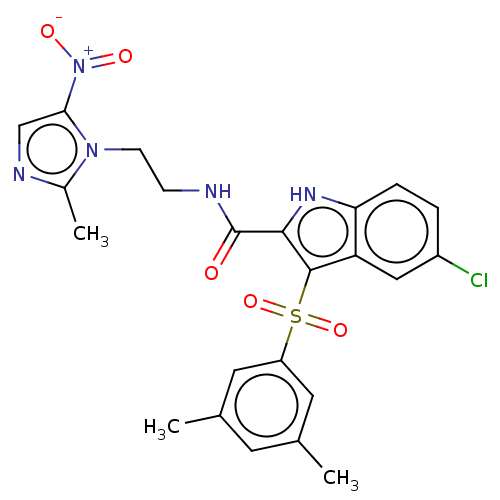 (CHEMBL3358174) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033863 (CHEMBL3358176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078887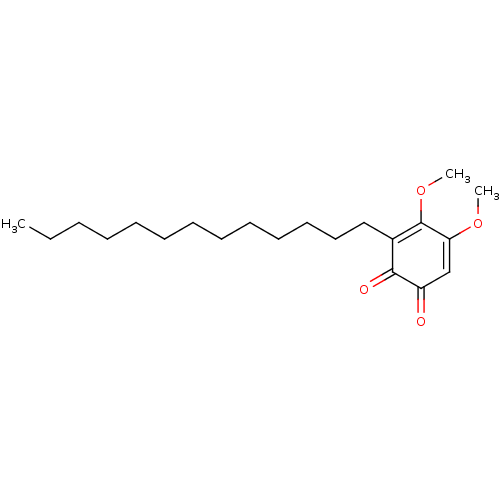 (CHEMBL3416360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033862 (CHEMBL3358175) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase V106A mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078889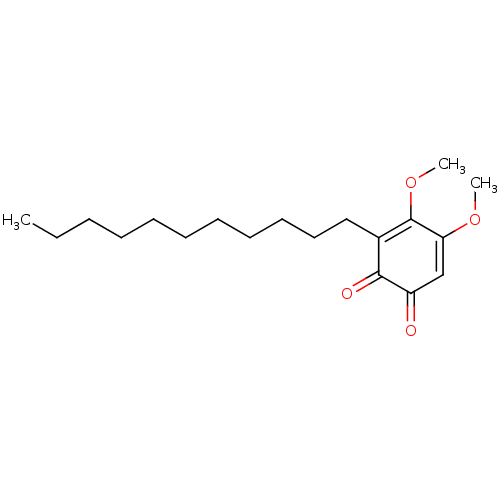 (CHEMBL3416358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078850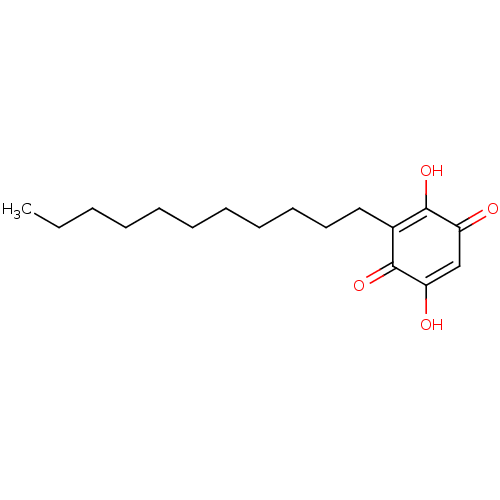 (CHEBI:4778 | Embelin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078850 (CHEBI:4778 | Embelin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human 5-LOX by cell free assay | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231998 (CHEMBL4085835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033866 (CHEMBL3358166) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50152151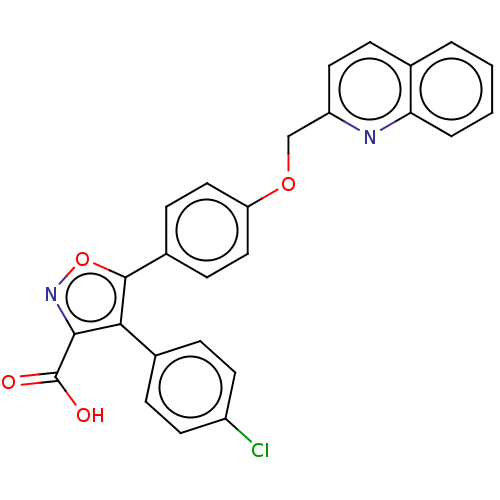 (CHEMBL3781006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "Luigi Vanvitelli" Curated by ChEMBL | Assay Description Inhibition of 5-LOX (unknown origin) | Eur J Med Chem 153: 65-72 (2018) Article DOI: 10.1016/j.ejmech.2017.10.020 BindingDB Entry DOI: 10.7270/Q26H4M0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM153384 (4-(4-fluorophenyl)-7-[[[5-[(2S)-1,1,1-trifluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "Luigi Vanvitelli" Curated by ChEMBL | Assay Description Inhibition of 5-LOX (unknown origin) | Eur J Med Chem 153: 65-72 (2018) Article DOI: 10.1016/j.ejmech.2017.10.020 BindingDB Entry DOI: 10.7270/Q26H4M0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50369677 (CHEMBL4172824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "Luigi Vanvitelli" Curated by ChEMBL | Assay Description Inhibition of 5-LOX (unknown origin) | Eur J Med Chem 153: 65-72 (2018) Article DOI: 10.1016/j.ejmech.2017.10.020 BindingDB Entry DOI: 10.7270/Q26H4M0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033885 (CHEMBL3263468) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase L100I mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078890 (CHEMBL3416357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033881 (CHEMBL3358173) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033884 (CHEMBL1766233) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50033880 (CHEMBL3358172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase K103N mutant assessed as reduction in enzyme activity | J Med Chem 57: 9945-57 (2014) Article DOI: 10.1021/jm5011622 BindingDB Entry DOI: 10.7270/Q2FB54K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 308 total ) | Next | Last >> |