 Found 269 hits with Last Name = 'agrawal' and Initial = 'ak'
Found 269 hits with Last Name = 'agrawal' and Initial = 'ak' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215294

(2-(N-(6-chloro-7-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CC(O)=O)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H12ClN3O6S/c1-5-3-6-9(15-12(20)11(19)14-6)10(8(5)13)16(4-7(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215284

(2-(N-(7-chloro-6-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CC(O)=O)S(C)(=O)=O Show InChI InChI=1S/C12H12ClN3O6S/c1-5-6(13)3-7-9(15-12(20)11(19)14-7)10(5)16(4-8(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215283
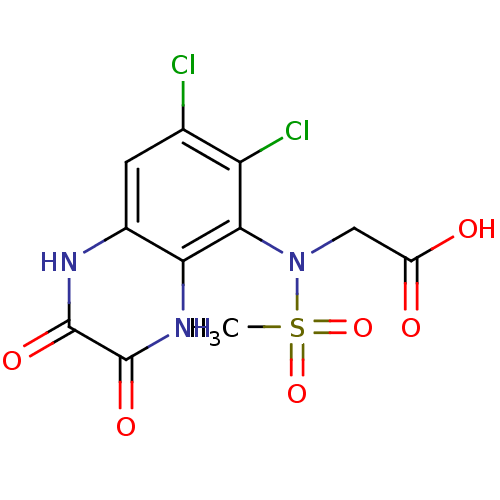
(2-(N-(6,7-dichloro-2,3-dioxo-1,2,3,4-tetrahydroqui...)Show SMILES CS(=O)(=O)N(CC(O)=O)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C11H9Cl2N3O6S/c1-23(21,22)16(3-6(17)18)9-7(13)4(12)2-5-8(9)15-11(20)10(19)14-5/h2H,3H2,1H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215282
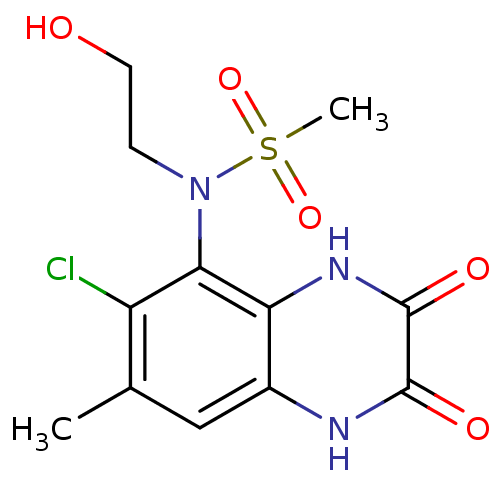
(CHEMBL429296 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CCO)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H14ClN3O5S/c1-6-5-7-9(15-12(19)11(18)14-7)10(8(6)13)16(3-4-17)22(2,20)21/h5,17H,3-4H2,1-2H3,(H,14,18)(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168554
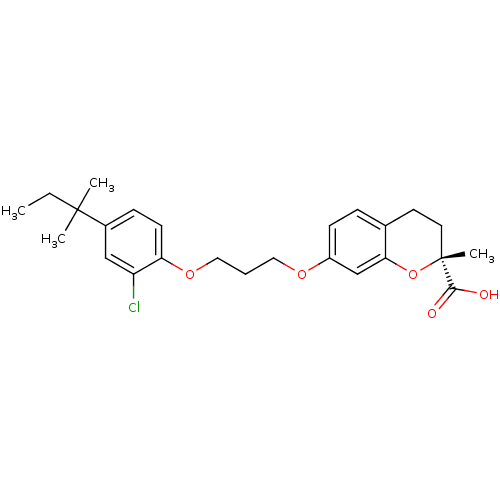
((R)-7-{3-[2-Chloro-4-(1,1-dimethyl-propyl)-phenoxy...)Show SMILES CCC(C)(C)c1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C25H31ClO5/c1-5-24(2,3)18-8-10-21(20(26)15-18)30-14-6-13-29-19-9-7-17-11-12-25(4,23(27)28)31-22(17)16-19/h7-10,15-16H,5-6,11-14H2,1-4H3,(H,27,28)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215286
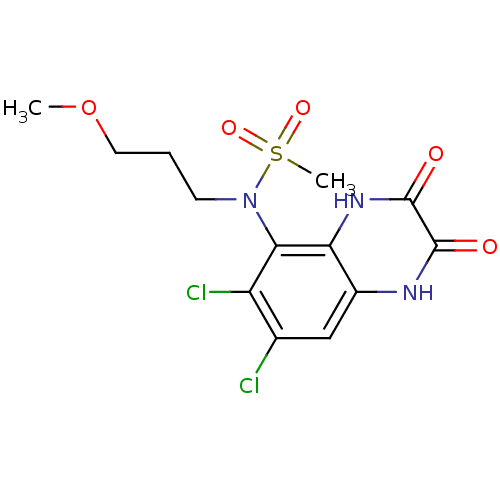
(CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H15Cl2N3O5S/c1-23-5-3-4-18(24(2,21)22)11-9(15)7(14)6-8-10(11)17-13(20)12(19)16-8/h6H,3-5H2,1-2H3,(H,16,19)(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215286

(CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H15Cl2N3O5S/c1-23-5-3-4-18(24(2,21)22)11-9(15)7(14)6-8-10(11)17-13(20)12(19)16-8/h6H,3-5H2,1-2H3,(H,16,19)(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281
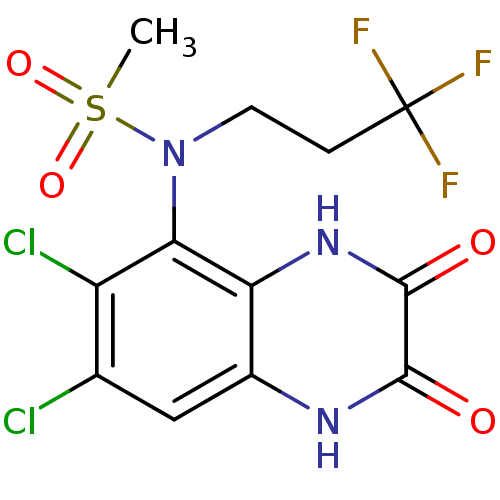
(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215287

(CHEMBL247035 | N-(3-methoxybenzyl)-N-(6,7-dichloro...)Show SMILES COc1cccc(CN(c2c(Cl)c(Cl)cc3[nH]c(=O)c(=O)[nH]c23)S(C)(=O)=O)c1 Show InChI InChI=1S/C17H15Cl2N3O5S/c1-27-10-5-3-4-9(6-10)8-22(28(2,25)26)15-13(19)11(18)7-12-14(15)21-17(24)16(23)20-12/h3-7H,8H2,1-2H3,(H,20,23)(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215296

(CHEMBL401849 | N-(7-chloro-6-methyl-2,3-dioxo-1,2,...)Show SMILES Cc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CCO)S(C)(=O)=O Show InChI InChI=1S/C12H14ClN3O5S/c1-6-7(13)5-8-9(15-12(19)11(18)14-8)10(6)16(3-4-17)22(2,20)21/h5,17H,3-4H2,1-2H3,(H,14,18)(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168544
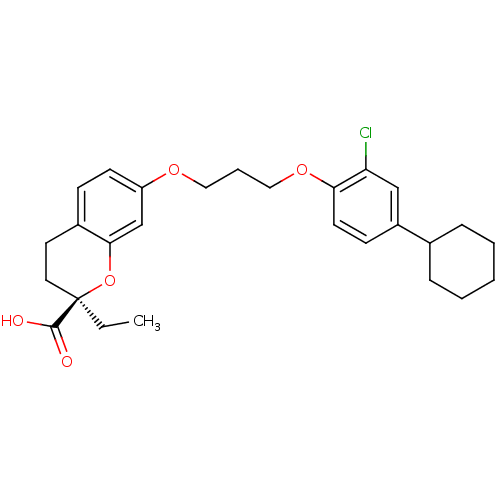
((R)-7-[3-(2-Chloro-4-cyclohexyl-phenoxy)-propoxy]-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C27H33ClO5/c1-2-27(26(29)30)14-13-20-9-11-22(18-25(20)33-27)31-15-6-16-32-24-12-10-21(17-23(24)28)19-7-4-3-5-8-19/h9-12,17-19H,2-8,13-16H2,1H3,(H,29,30)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168557

((R)-7-[3-(2-Chloro-4-isobutyl-phenoxy)-propoxy]-2-...)Show SMILES CC(C)Cc1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H29ClO5/c1-16(2)13-17-5-8-21(20(25)14-17)29-12-4-11-28-19-7-6-18-9-10-24(3,23(26)27)30-22(18)15-19/h5-8,14-16H,4,9-13H2,1-3H3,(H,26,27)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215285

(CHEMBL400508 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCOCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C14H17Cl2N3O5S/c1-3-24-6-4-5-19(25(2,22)23)12-10(16)8(15)7-9-11(12)18-14(21)13(20)17-9/h7H,3-6H2,1-2H3,(H,17,20)(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215276

(CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C13H12Cl2F3N3O4S/c1-2-26(24,25)21(4-3-13(16,17)18)10-8(15)6(14)5-7-9(10)20-12(23)11(22)19-7/h5H,2-4H2,1H3,(H,19,22)(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281

(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281

(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168543
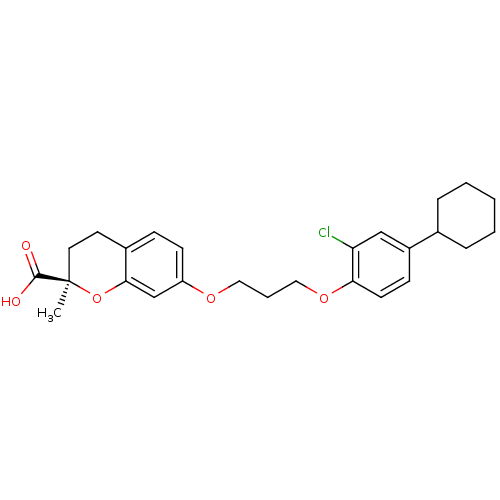
((R)-7-[3-(2-Chloro-4-cyclohexyl-phenoxy)-propoxy]-...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C26H31ClO5/c1-26(25(28)29)13-12-19-8-10-21(17-24(19)32-26)30-14-5-15-31-23-11-9-20(16-22(23)27)18-6-3-2-4-7-18/h8-11,16-18H,2-7,12-15H2,1H3,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168555
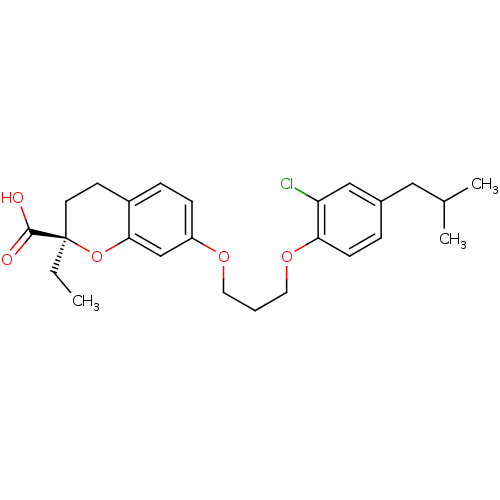
((R)-7-[3-(2-Chloro-4-isobutyl-phenoxy)-propoxy]-2-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(CC(C)C)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C25H31ClO5/c1-4-25(24(27)28)11-10-19-7-8-20(16-23(19)31-25)29-12-5-13-30-22-9-6-18(14-17(2)3)15-21(22)26/h6-9,15-17H,4-5,10-14H2,1-3H3,(H,27,28)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168572

((R)-7-[3-(2-Chloro-4-trifluoromethoxy-phenoxy)-pro...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(OC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O6/c1-2-21(20(27)28)9-8-14-4-5-15(13-19(14)32-21)29-10-3-11-30-18-7-6-16(12-17(18)23)31-22(24,25)26/h4-7,12-13H,2-3,8-11H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168567
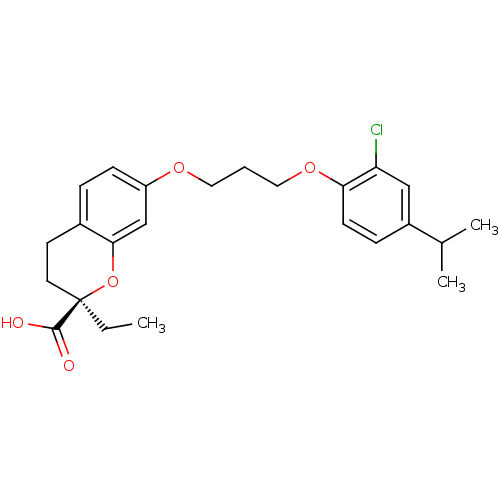
((R)-7-[3-(2-Chloro-4-isopropyl-phenoxy)-propoxy]-2...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(C)C)cc2O1)C(O)=O Show InChI InChI=1S/C24H29ClO5/c1-4-24(23(26)27)11-10-17-6-8-19(15-22(17)30-24)28-12-5-13-29-21-9-7-18(16(2)3)14-20(21)25/h6-9,14-16H,4-5,10-13H2,1-3H3,(H,26,27)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215279
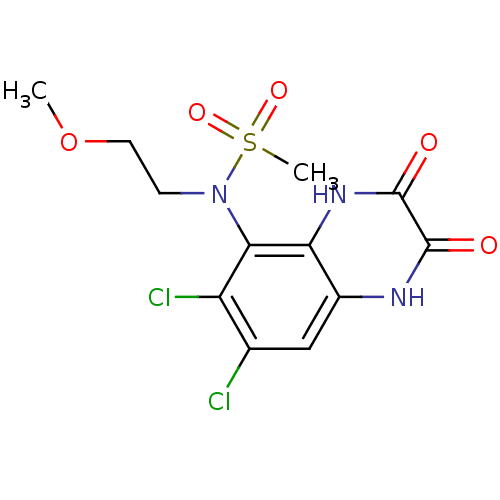
(CHEMBL248439 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C12H13Cl2N3O5S/c1-22-4-3-17(23(2,20)21)10-8(14)6(13)5-7-9(10)16-12(19)11(18)15-7/h5H,3-4H2,1-2H3,(H,15,18)(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215298

(CHEMBL399075 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CC1CCCCO1)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 |w:6.5| Show InChI InChI=1S/C15H17Cl2N3O5S/c1-26(23,24)20(7-8-4-2-3-5-25-8)13-11(17)9(16)6-10-12(13)19-15(22)14(21)18-10/h6,8H,2-5,7H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215297
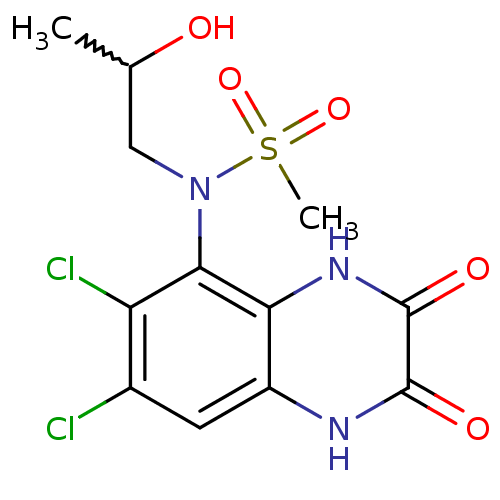
(CHEMBL247034 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CC(O)CN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O |w:1.0| Show InChI InChI=1S/C12H13Cl2N3O5S/c1-5(18)4-17(23(2,21)22)10-8(14)6(13)3-7-9(10)16-12(20)11(19)15-7/h3,5,18H,4H2,1-2H3,(H,15,19)(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168560

((R)-7-[3-(2-Chloro-4-cyclopentyl-phenoxy)-propoxy]...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C25H29ClO5/c1-25(24(27)28)12-11-18-7-9-20(16-23(18)31-25)29-13-4-14-30-22-10-8-19(15-21(22)26)17-5-2-3-6-17/h7-10,15-17H,2-6,11-14H2,1H3,(H,27,28)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215292

(CHEMBL401100 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCCO)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H13Cl2N3O5S/c1-23(21,22)17(3-2-4-18)10-8(14)6(13)5-7-9(10)16-12(20)11(19)15-7/h5,18H,2-4H2,1H3,(H,15,19)(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215280

(CHEMBL248436 | N-(3-fluorobenzyl)-N-(6,7-dichloro-...)Show SMILES CS(=O)(=O)N(Cc1cccc(F)c1)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C16H12Cl2FN3O4S/c1-27(25,26)22(7-8-3-2-4-9(19)5-8)14-12(18)10(17)6-11-13(14)21-16(24)15(23)20-11/h2-6H,7H2,1H3,(H,20,23)(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168558
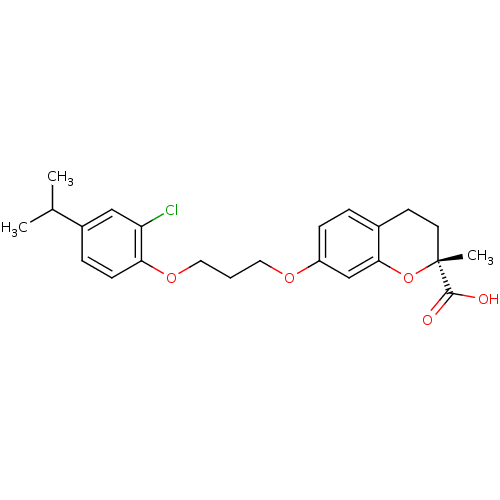
((R)-7-[3-(2-Chloro-4-isopropyl-phenoxy)-propoxy]-2...)Show SMILES CC(C)c1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H27ClO5/c1-15(2)17-6-8-20(19(24)13-17)28-12-4-11-27-18-7-5-16-9-10-23(3,22(25)26)29-21(16)14-18/h5-8,13-15H,4,9-12H2,1-3H3,(H,25,26)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215278
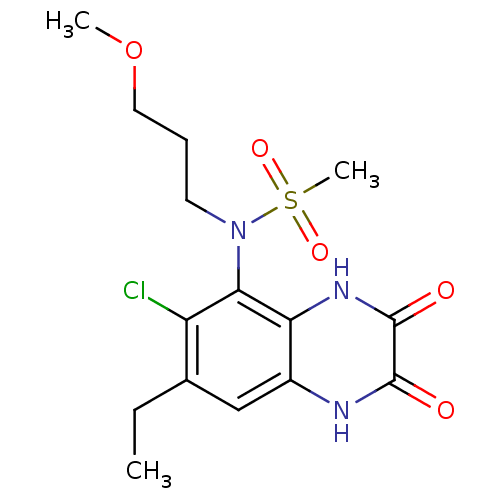
(CHEMBL248446 | N-(6-chloro-7-ethyl-2,3-dioxo-1,2,3...)Show SMILES CCc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CCCOC)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C15H20ClN3O5S/c1-4-9-8-10-12(18-15(21)14(20)17-10)13(11(9)16)19(25(3,22)23)6-5-7-24-2/h8H,4-7H2,1-3H3,(H,17,20)(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168547
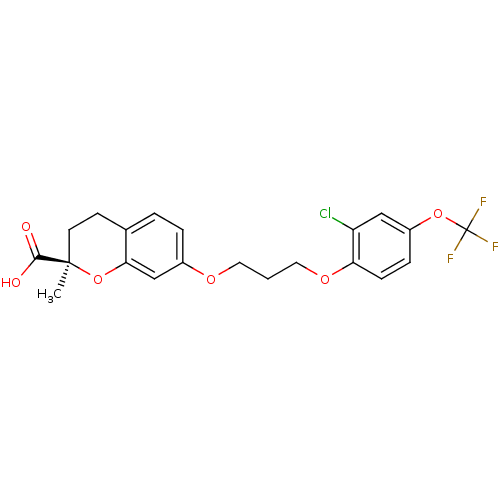
((R)-7-[3-(2-Chloro-4-trifluoromethoxy-phenoxy)-pro...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(OC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C21H20ClF3O6/c1-20(19(26)27)8-7-13-3-4-14(12-18(13)31-20)28-9-2-10-29-17-6-5-15(11-16(17)22)30-21(23,24)25/h3-6,11-12H,2,7-10H2,1H3,(H,26,27)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215277

(CHEMBL401253 | N-(7-chloro-6-ethyl-2,3-dioxo-1,2,3...)Show SMILES CCc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CCO)S(C)(=O)=O Show InChI InChI=1S/C13H16ClN3O5S/c1-3-7-8(14)6-9-10(16-13(20)12(19)15-9)11(7)17(4-5-18)23(2,21)22/h6,18H,3-5H2,1-2H3,(H,15,19)(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215295
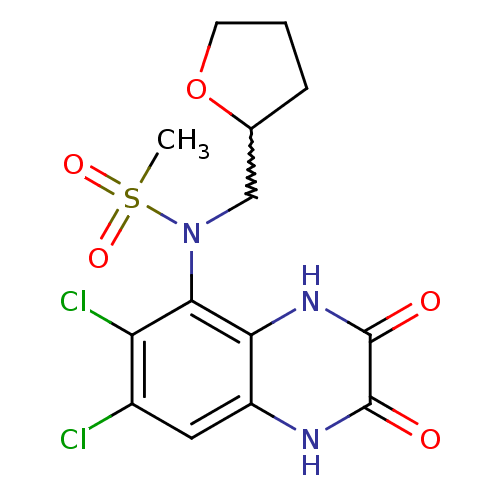
(CHEMBL248438 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CC1CCCO1)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 |w:6.5| Show InChI InChI=1S/C14H15Cl2N3O5S/c1-25(22,23)19(6-7-3-2-4-24-7)12-10(16)8(15)5-9-11(12)18-14(21)13(20)17-9/h5,7H,2-4,6H2,1H3,(H,17,20)(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148090
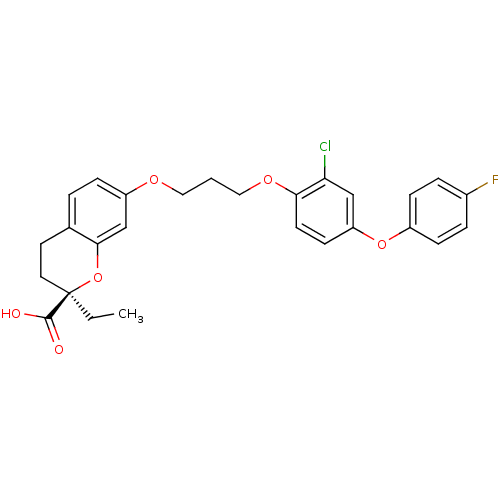
((R)-7-{3-[2-Chloro-4-(4-fluoro-phenoxy)-phenoxy]-p...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(Oc4ccc(F)cc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C27H26ClFO6/c1-2-27(26(30)31)13-12-18-4-7-21(17-25(18)35-27)32-14-3-15-33-24-11-10-22(16-23(24)28)34-20-8-5-19(29)6-9-20/h4-11,16-17H,2-3,12-15H2,1H3,(H,30,31)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148110
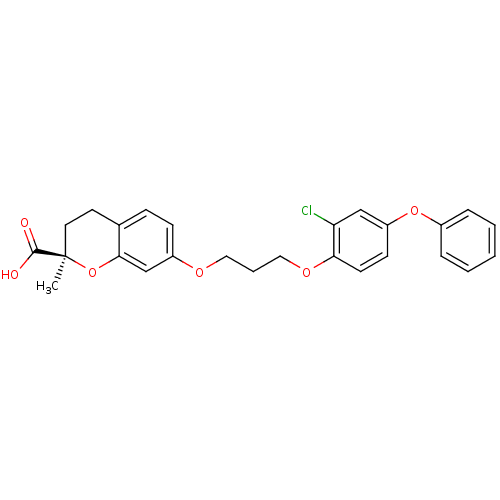
((R)-7-[3-(2-Chloro-4-phenoxy-phenoxy)-propoxy]-2-m...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-26(25(28)29)13-12-18-8-9-20(17-24(18)33-26)30-14-5-15-31-23-11-10-21(16-22(23)27)32-19-6-3-2-4-7-19/h2-4,6-11,16-17H,5,12-15H2,1H3,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148090

((R)-7-{3-[2-Chloro-4-(4-fluoro-phenoxy)-phenoxy]-p...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(Oc4ccc(F)cc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C27H26ClFO6/c1-2-27(26(30)31)13-12-18-4-7-21(17-25(18)35-27)32-14-3-15-33-24-11-10-22(16-23(24)28)34-20-8-5-19(29)6-9-20/h4-11,16-17H,2-3,12-15H2,1H3,(H,30,31)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168553
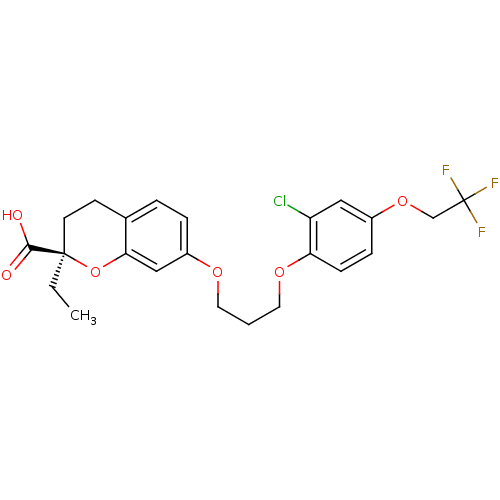
((R)-7-{3-[2-Chloro-4-(2,2,2-trifluoro-ethoxy)-phen...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(OCC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C23H24ClF3O6/c1-2-22(21(28)29)9-8-15-4-5-17(13-20(15)33-22)30-10-3-11-31-19-7-6-16(12-18(19)24)32-14-23(25,26)27/h4-7,12-13H,2-3,8-11,14H2,1H3,(H,28,29)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215291
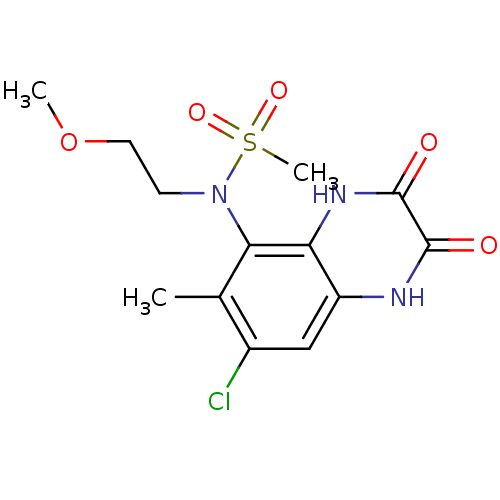
(CHEMBL248445 | N-(7-chloro-6-methyl-2,3-dioxo-1,2,...)Show SMILES COCCN(c1c(C)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H16ClN3O5S/c1-7-8(14)6-9-10(16-13(19)12(18)15-9)11(7)17(4-5-22-2)23(3,20)21/h6H,4-5H2,1-3H3,(H,15,18)(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215288
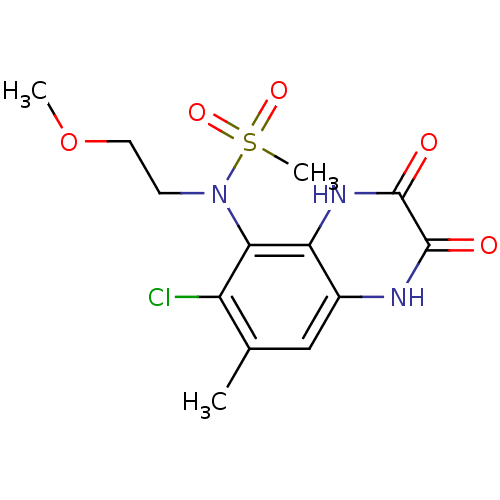
(CHEMBL248444 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...)Show SMILES COCCN(c1c(Cl)c(C)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H16ClN3O5S/c1-7-6-8-10(16-13(19)12(18)15-8)11(9(7)14)17(4-5-22-2)23(3,20)21/h6H,4-5H2,1-3H3,(H,15,18)(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168565

((R)-7-[3-(2-Chloro-4-trifluoromethyl-phenoxy)-prop...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(F)(F)F)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O5/c1-2-21(20(27)28)9-8-14-4-6-16(13-19(14)31-21)29-10-3-11-30-18-7-5-15(12-17(18)23)22(24,25)26/h4-7,12-13H,2-3,8-11H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215276

(CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C13H12Cl2F3N3O4S/c1-2-26(24,25)21(4-3-13(16,17)18)10-8(15)6(14)5-7-9(10)20-12(23)11(22)19-7/h5H,2-4H2,1H3,(H,19,22)(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215276

(CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C13H12Cl2F3N3O4S/c1-2-26(24,25)21(4-3-13(16,17)18)10-8(15)6(14)5-7-9(10)20-12(23)11(22)19-7/h5H,2-4H2,1H3,(H,19,22)(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168571
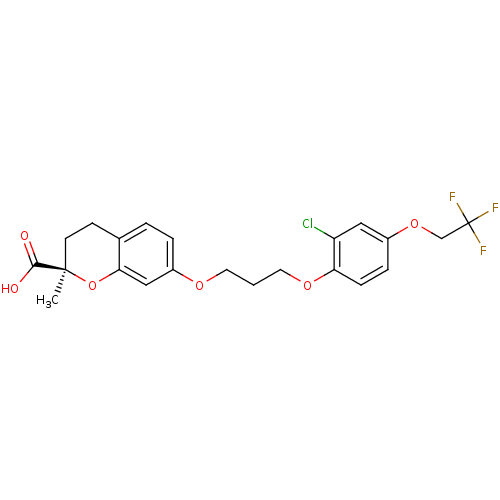
((R)-7-{3-[2-Chloro-4-(2,2,2-trifluoro-ethoxy)-phen...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(OCC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O6/c1-21(20(27)28)8-7-14-3-4-16(12-19(14)32-21)29-9-2-10-30-18-6-5-15(11-17(18)23)31-13-22(24,25)26/h3-6,11-12H,2,7-10,13H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148092
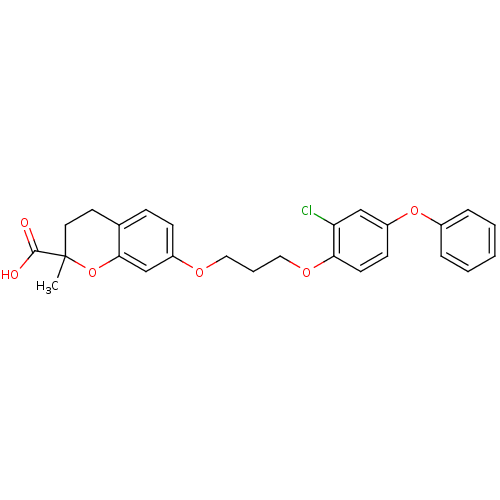
(7-[3-(2-Chloro-4-phenoxy-phenoxy)-propoxy]-2-methy...)Show SMILES CC1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-26(25(28)29)13-12-18-8-9-20(17-24(18)33-26)30-14-5-15-31-23-11-10-21(16-22(23)27)32-19-6-3-2-4-7-19/h2-4,6-11,16-17H,5,12-15H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148097

(7-[3-(2-Chloro-4-phenoxy-phenoxy)-propoxy]-2-ethyl...)Show SMILES CCC1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C27H27ClO6/c1-2-27(26(29)30)14-13-19-9-10-21(18-25(19)34-27)31-15-6-16-32-24-12-11-22(17-23(24)28)33-20-7-4-3-5-8-20/h3-5,7-12,17-18H,2,6,13-16H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168545
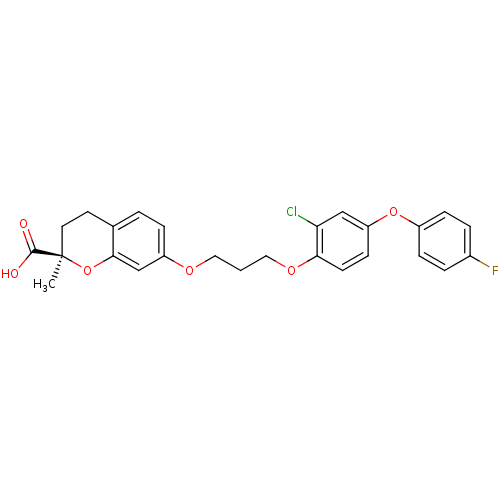
((R)-7-{3-[2-Chloro-4-(4-fluoro-phenoxy)-phenoxy]-p...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(Oc4ccc(F)cc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C26H24ClFO6/c1-26(25(29)30)12-11-17-3-6-20(16-24(17)34-26)31-13-2-14-32-23-10-9-21(15-22(23)27)33-19-7-4-18(28)5-8-19/h3-10,15-16H,2,11-14H2,1H3,(H,29,30)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215290

(CHEMBL399274 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCOCCOCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C15H19Cl2N3O6S/c1-3-25-6-7-26-5-4-20(27(2,23)24)13-11(17)9(16)8-10-12(13)19-15(22)14(21)18-10/h8H,3-7H2,1-2H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168556
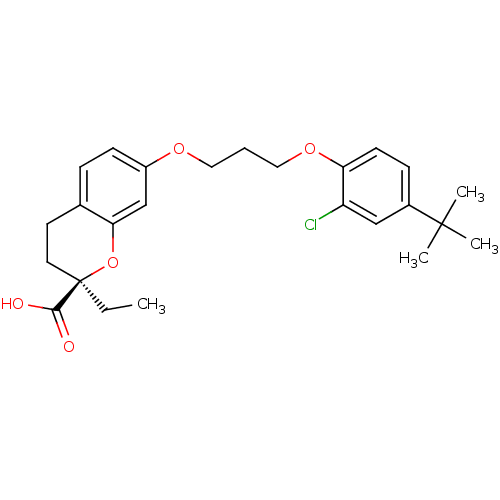
((R)-7-[3-(4-tert-Butyl-2-chloro-phenoxy)-propoxy]-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(C)(C)C)cc2O1)C(O)=O Show InChI InChI=1S/C25H31ClO5/c1-5-25(23(27)28)12-11-17-7-9-19(16-22(17)31-25)29-13-6-14-30-21-10-8-18(15-20(21)26)24(2,3)4/h7-10,15-16H,5-6,11-14H2,1-4H3,(H,27,28)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human PPAR gamma receptor using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215293
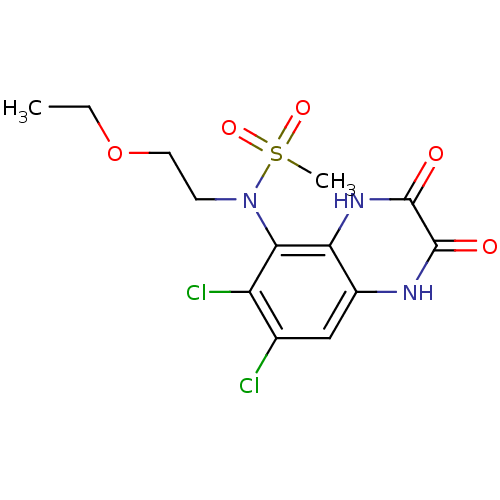
(CHEMBL248442 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCOCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H15Cl2N3O5S/c1-3-23-5-4-18(24(2,21)22)11-9(15)7(14)6-8-10(11)17-13(20)12(19)16-8/h6H,3-5H2,1-2H3,(H,16,19)(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168546
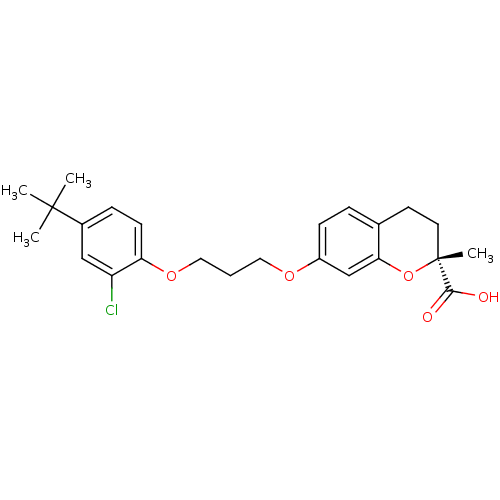
((R)-7-[3-(4-tert-Butyl-2-chloro-phenoxy)-propoxy]-...)Show SMILES CC(C)(C)c1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H29ClO5/c1-23(2,3)17-7-9-20(19(25)14-17)29-13-5-12-28-18-8-6-16-10-11-24(4,22(26)27)30-21(16)15-18/h6-9,14-15H,5,10-13H2,1-4H3,(H,26,27)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168548

((R)-7-[3-(2-Chloro-4-isopropoxy-phenoxy)-propoxy]-...)Show SMILES CC(C)Oc1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H27ClO6/c1-15(2)29-18-7-8-20(19(24)13-18)28-12-4-11-27-17-6-5-16-9-10-23(3,22(25)26)30-21(16)14-17/h5-8,13-15H,4,9-12H2,1-3H3,(H,25,26)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data