

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475465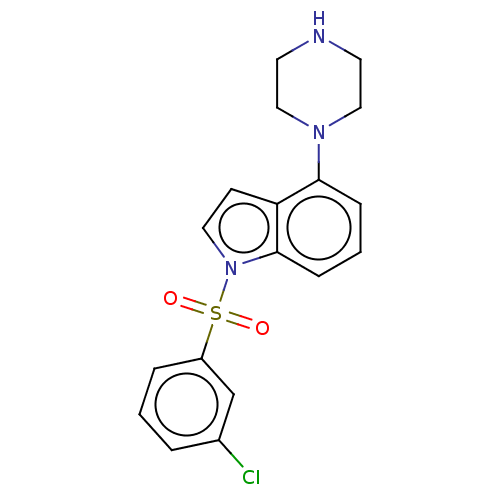 (CHEMBL196410) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475462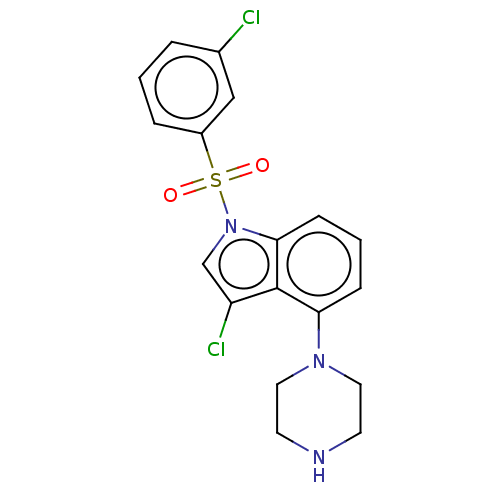 (CHEMBL371375) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475480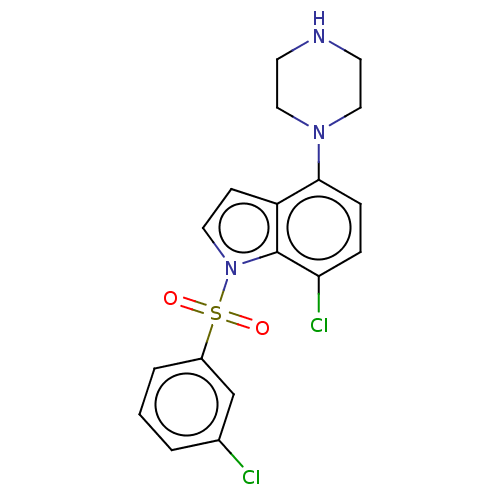 (CHEMBL193629) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50174269 (1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475467 (CHEMBL425015) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475475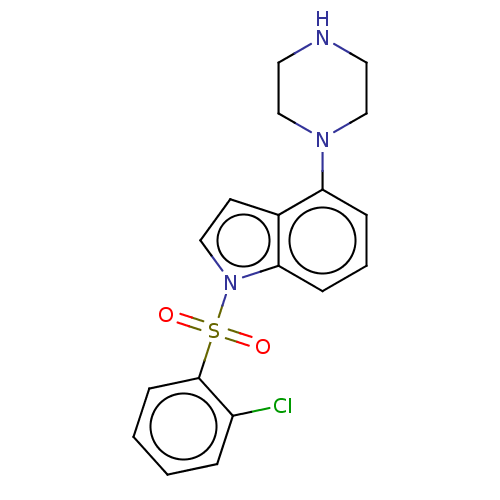 (CHEMBL372513) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475477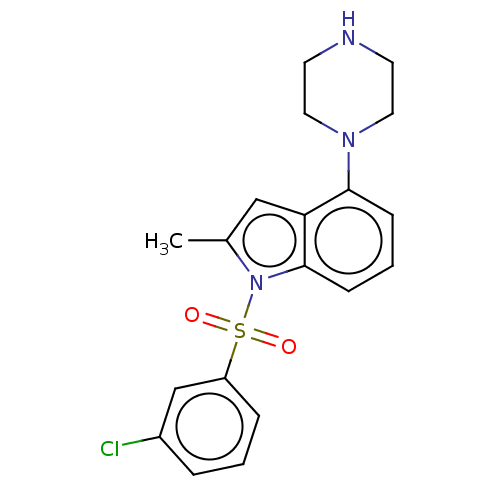 (CHEMBL372929) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044607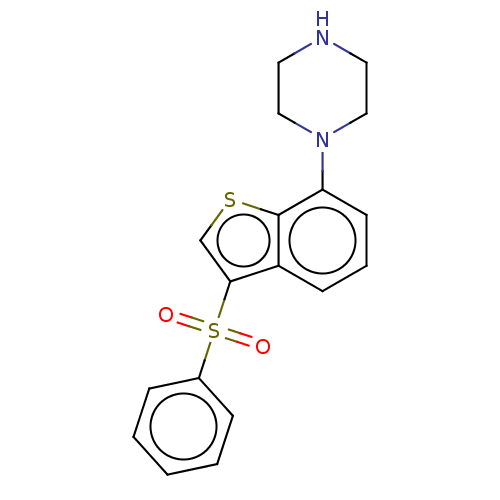 (CHEMBL372537) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475463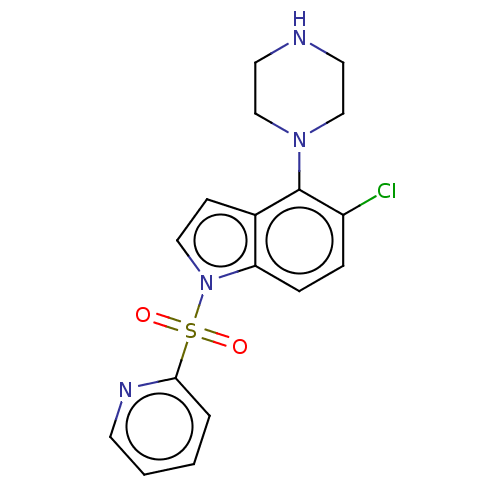 (CHEMBL194915) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475473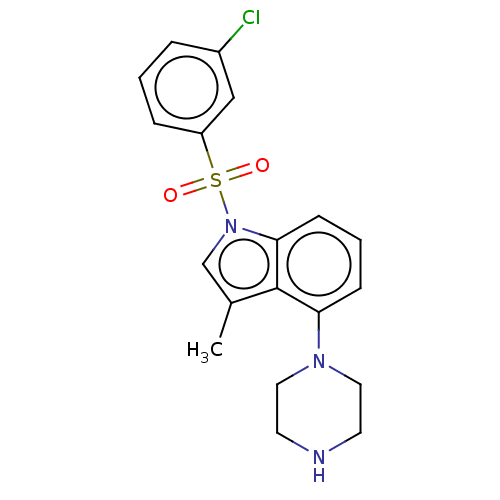 (CHEMBL194039) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475479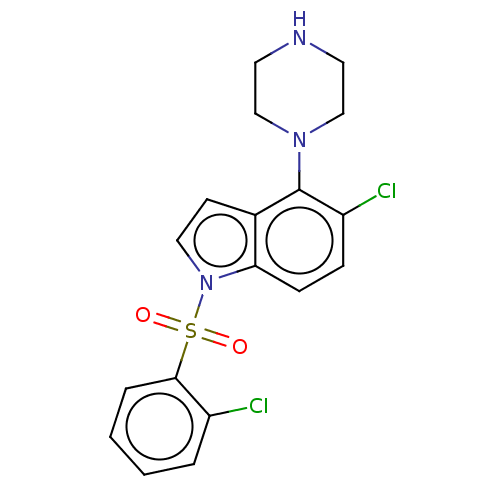 (CHEMBL371176) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475470 (CHEMBL370209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475464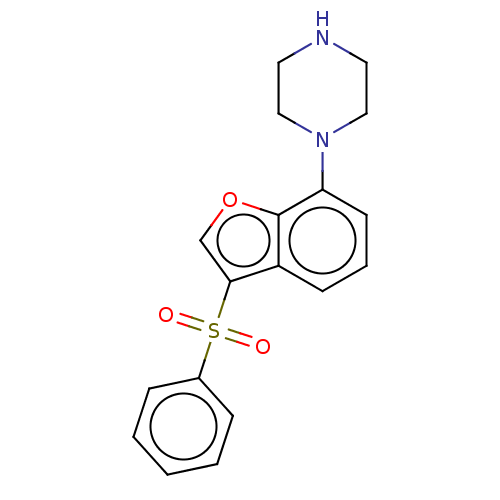 (CHEMBL197574) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM28583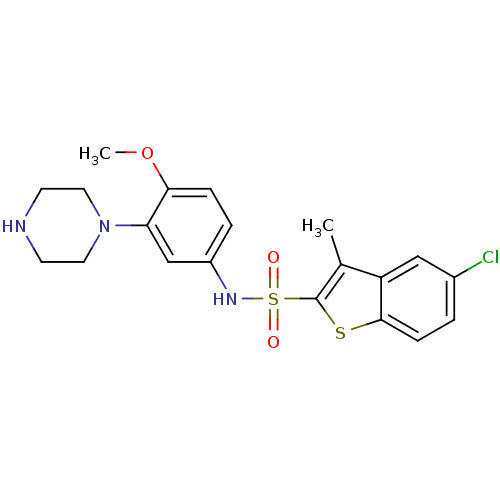 (5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475466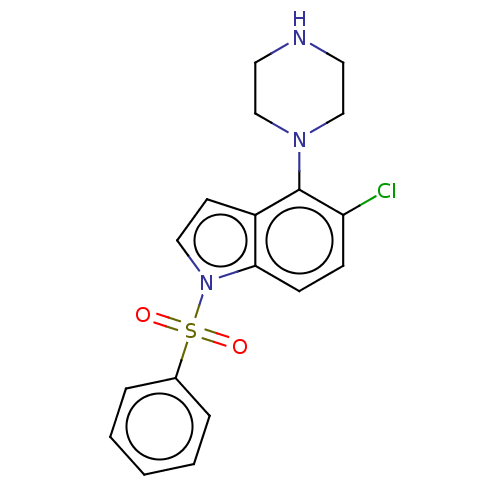 (CHEMBL193665) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475471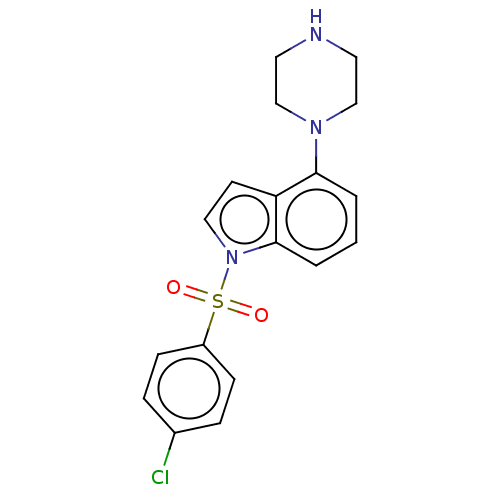 (CHEMBL371876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475481 (CHEMBL197297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044623 (CHEMBL193400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475478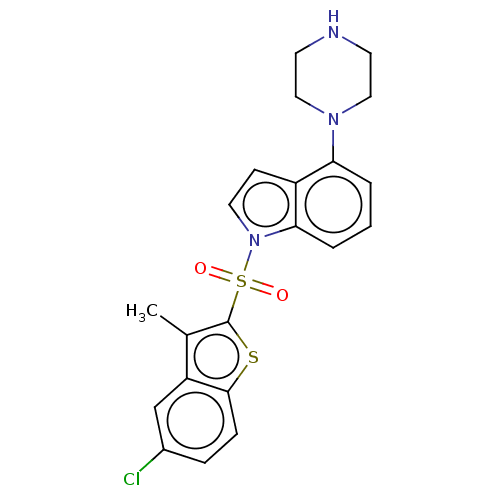 (CHEMBL196644) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475482 (CHEMBL193379) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475472 (CHEMBL372287) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044623 (CHEMBL193400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 6 receptor of human caudate | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475476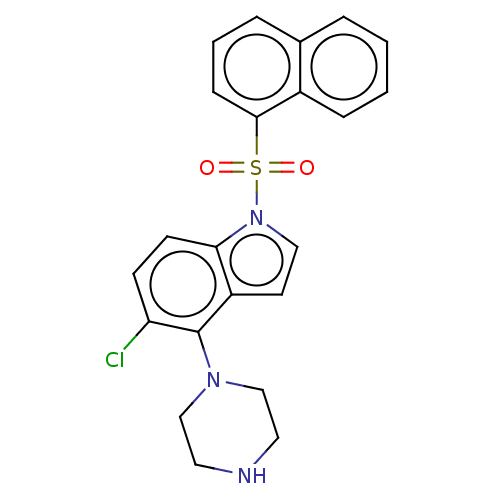 (CHEMBL196103) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475474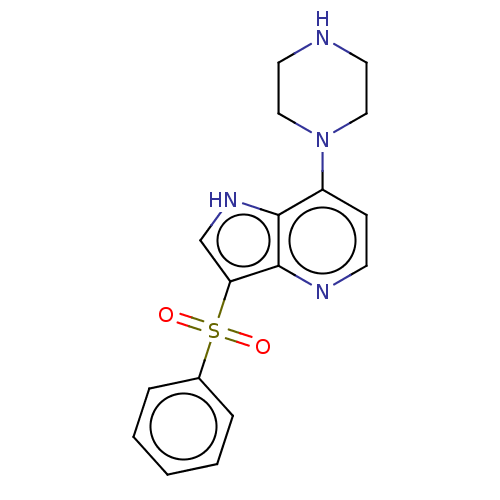 (CHEMBL426640) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321113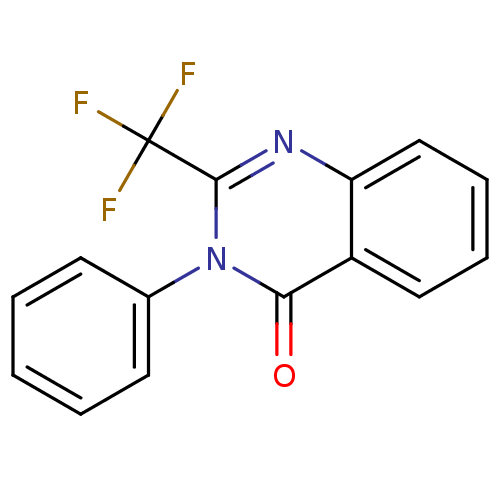 (3-Phenyl-2-(trifluoromethyl)quinazolin-4(3H)-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50044623 (CHEMBL193400) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 6 receptor of rat striatum | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475469 (CHEMBL196524) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50475468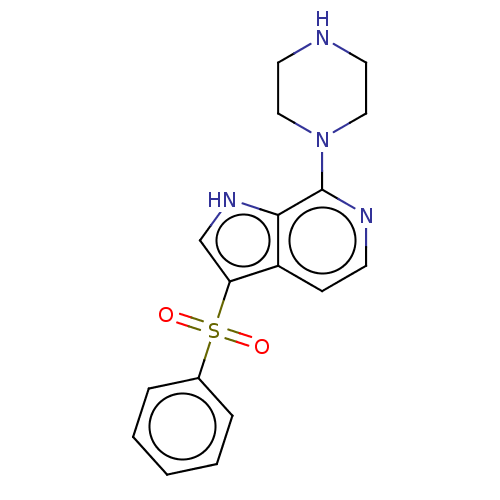 (CHEMBL366248) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells | Bioorg Med Chem Lett 15: 4867-71 (2005) Article DOI: 10.1016/j.bmcl.2005.06.107 BindingDB Entry DOI: 10.7270/Q2028V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068225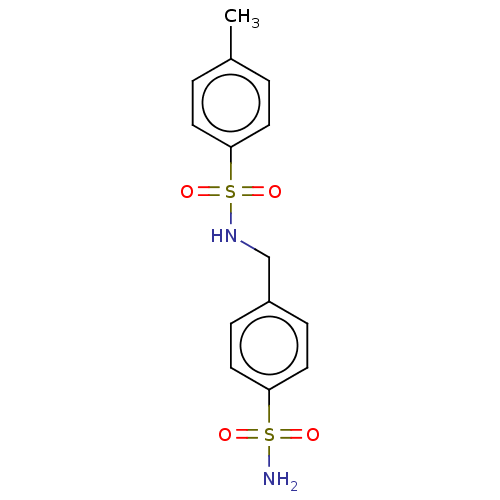 (CHEMBL3403324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068229 (CHEMBL3403327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50428400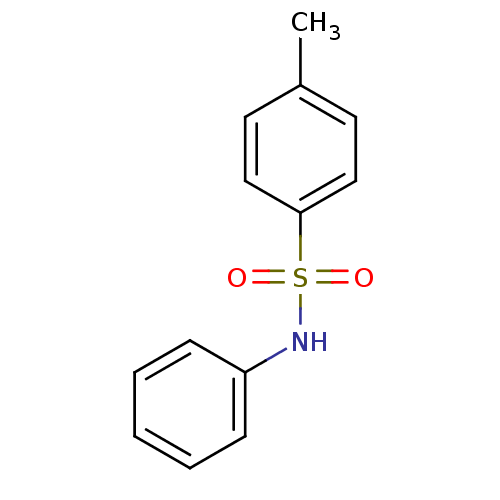 (CA inhibitor, 2 | CHEMBL182659 | [(4-Methylphenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068230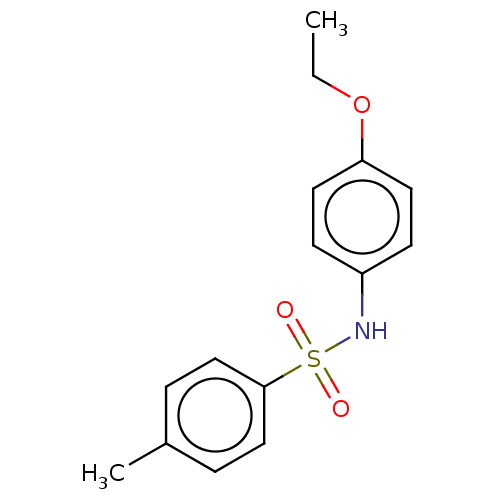 (CHEMBL3403329) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321123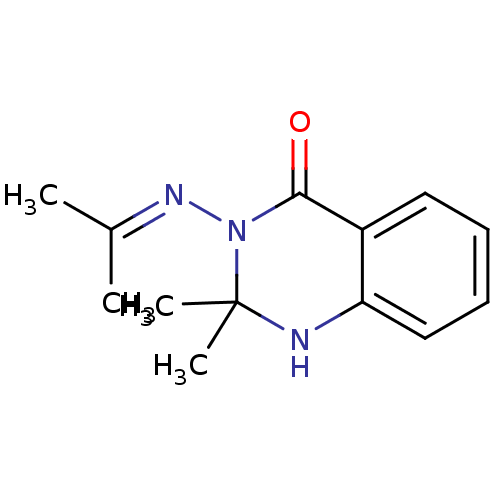 (3-(Isopropylideneamino)-2,2-dimethyl-2,3-dihydroqu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068220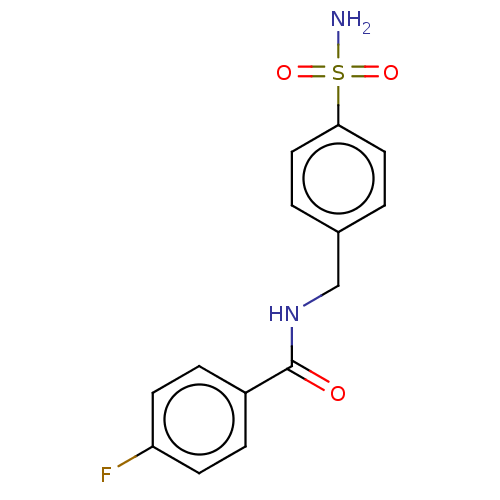 (CHEMBL3403320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068228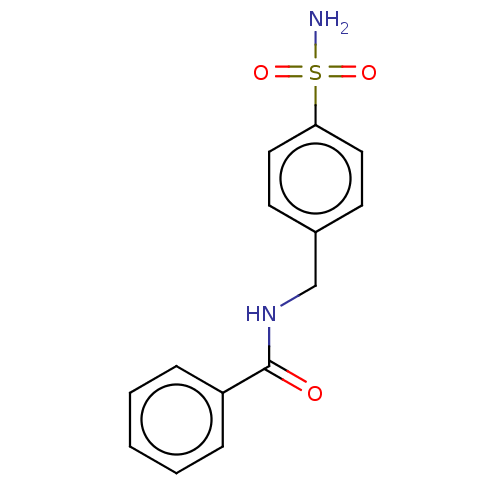 (CHEMBL3403317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 918 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068227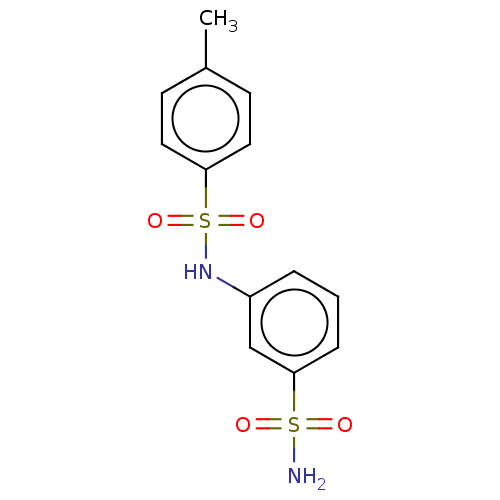 (CHEMBL3403325) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321122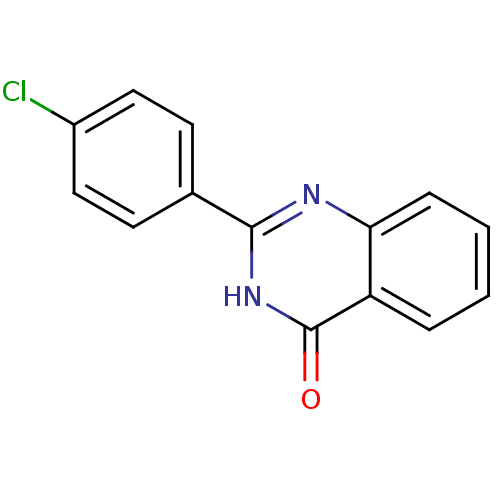 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50247721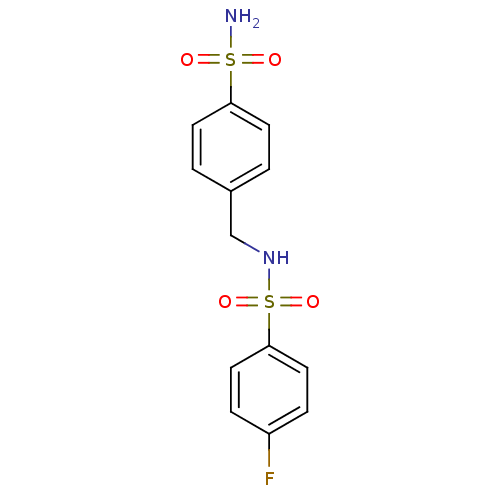 (4-fluoro-N-(4-sulfamoylbenzyl)benzenesulfonamide |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM16661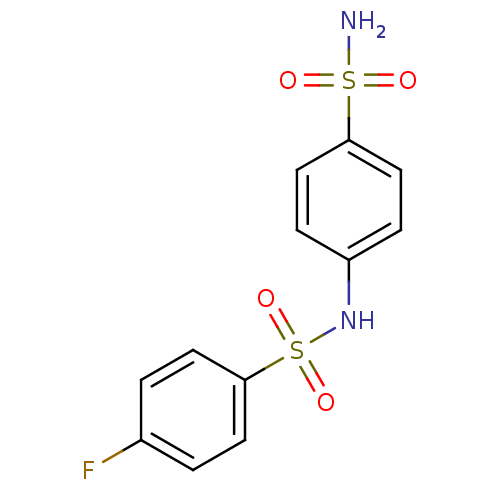 (1-N-(4-fluorobenzene)benzene-1,4-disulfonamide | a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50241179 ((S)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068218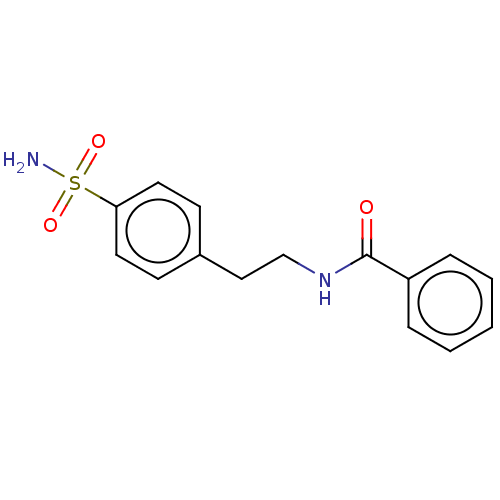 (CHEMBL3403318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068221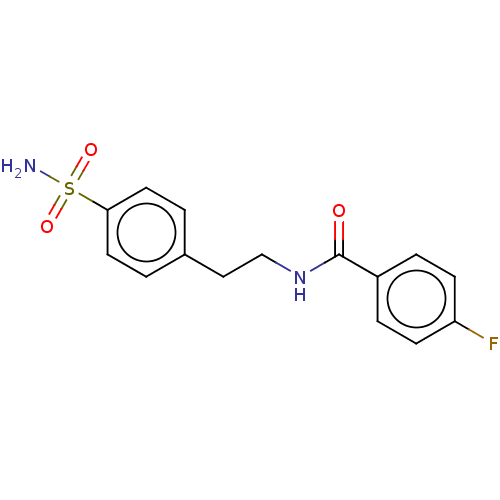 (CHEMBL3403321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068222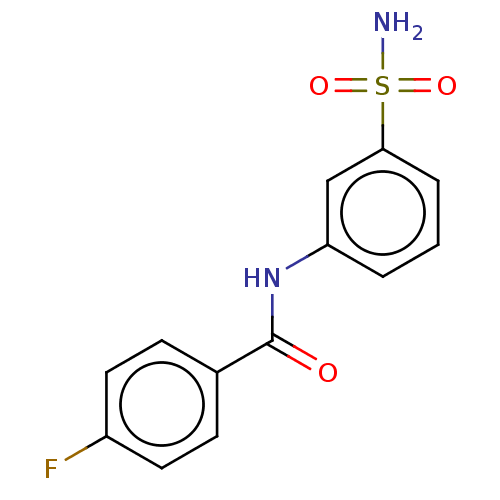 (CHEMBL3403322) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM16652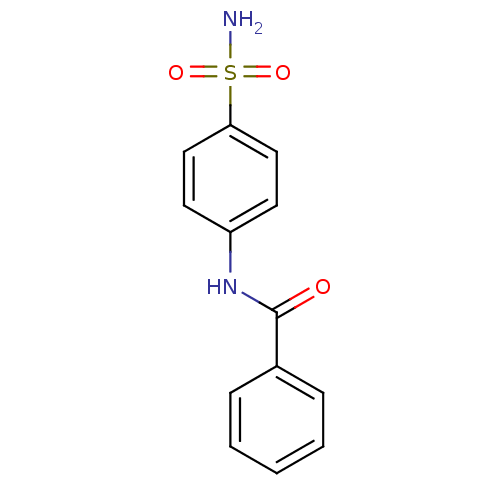 (4-Benzoylamino-benzenesulfonamide | CHEMBL23559 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068226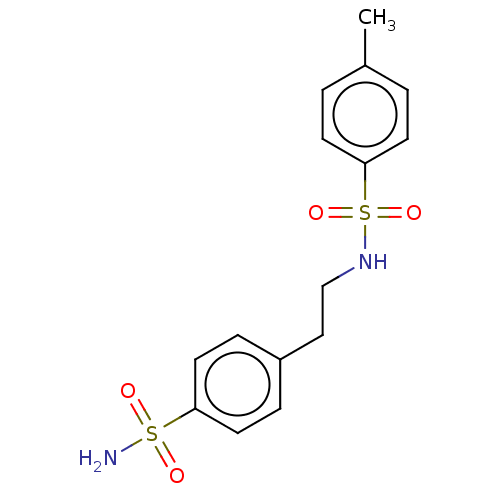 (CHEMBL1607835) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068219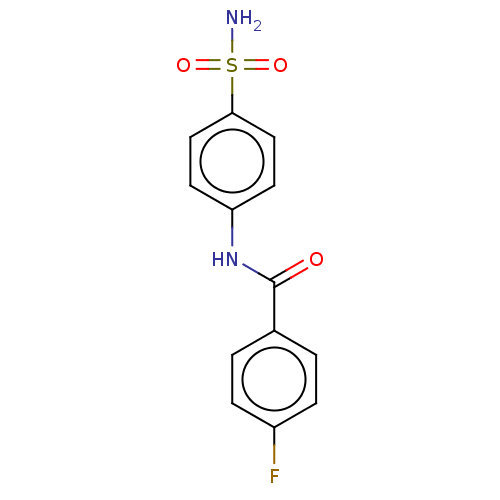 (CHEMBL3403319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321112 (3-Amino-2-(trifluoromethyl)quinazolin-4(3H)-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321118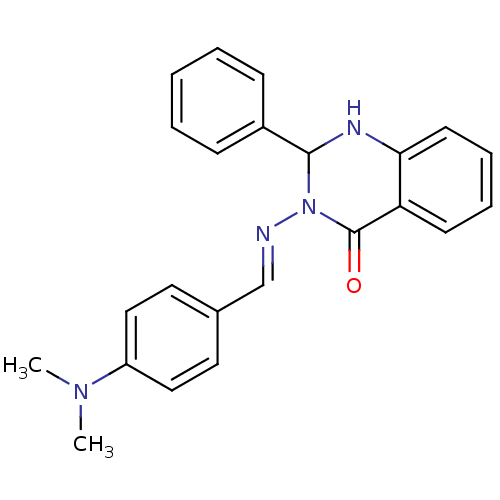 (3-({(1E)-[4-(Dimethylamino)phenyl]methylene}amino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321114 (3-Amino-2-methylquinazolin-4(3H)-one | CHEMBL11631...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321115 (2-(4-Methylphenyl)-3-(1,3-thiazol-2-yl)quinazolin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 5.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 490 total ) | Next | Last >> |