

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50004154 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004155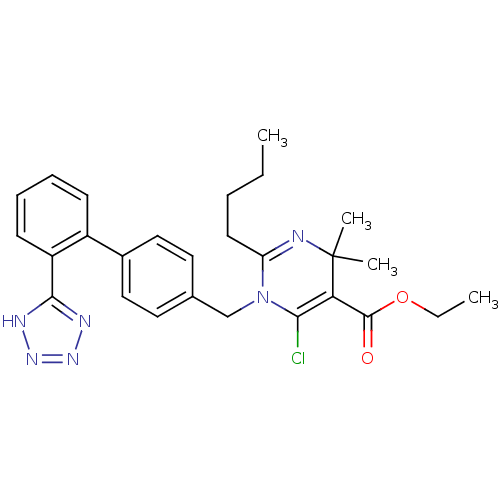 (2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004164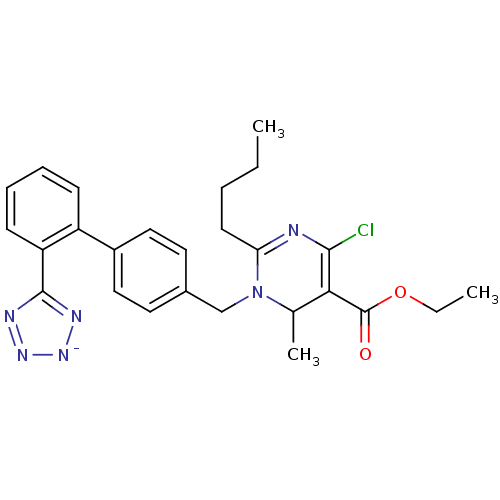 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50406795 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004153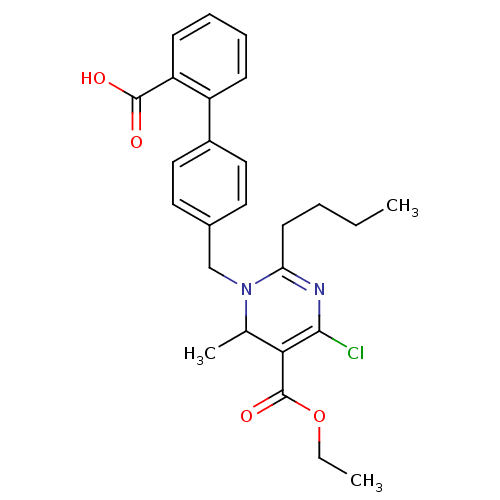 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Homo sapiens (Human)) | BDBM50281270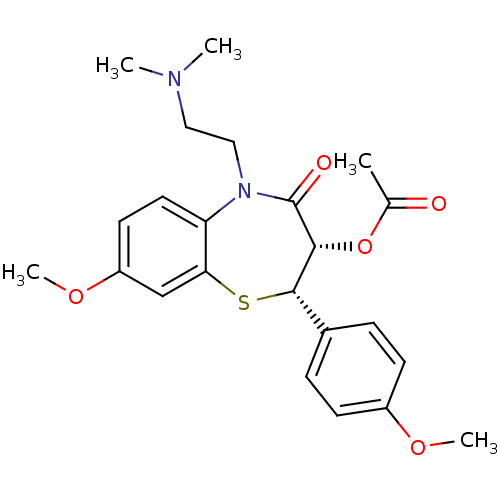 (Acetic acid (2S,3S)-5-(2-dimethylamino-ethyl)-8-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of guinea pig striated muscle | Bioorg Med Chem Lett 3: 2797-2800 (1993) Article DOI: 10.1016/S0960-894X(01)80767-8 BindingDB Entry DOI: 10.7270/Q2FN164V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004160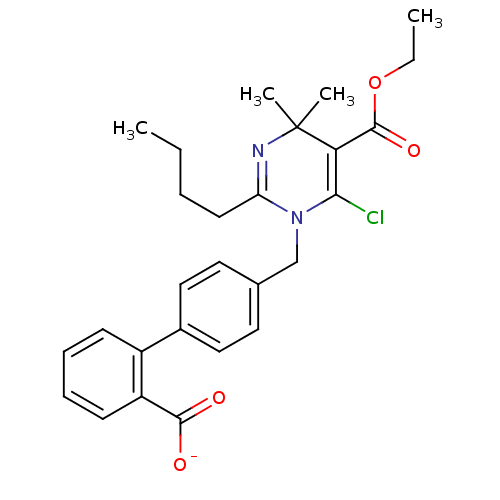 (CHEMBL145036 | Sodium; 4'-(2-butyl-6-chloro-5-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004163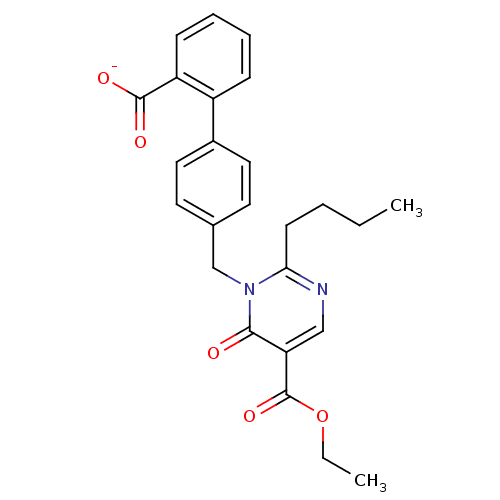 (CHEMBL342287 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Homo sapiens (Human)) | BDBM50281272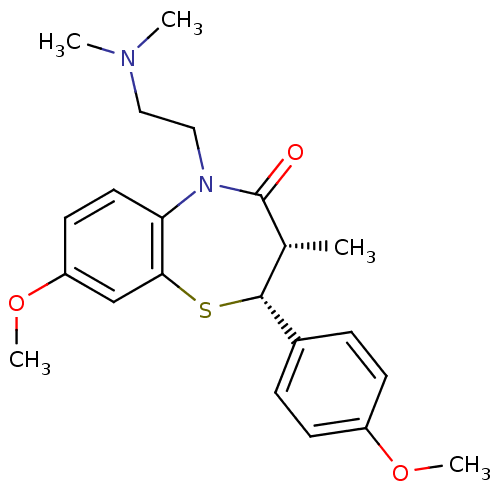 ((2R,3S)-5-(2-Dimethylamino-ethyl)-8-methoxy-2-(4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of guinea pig striated muscle | Bioorg Med Chem Lett 3: 2797-2800 (1993) Article DOI: 10.1016/S0960-894X(01)80767-8 BindingDB Entry DOI: 10.7270/Q2FN164V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004162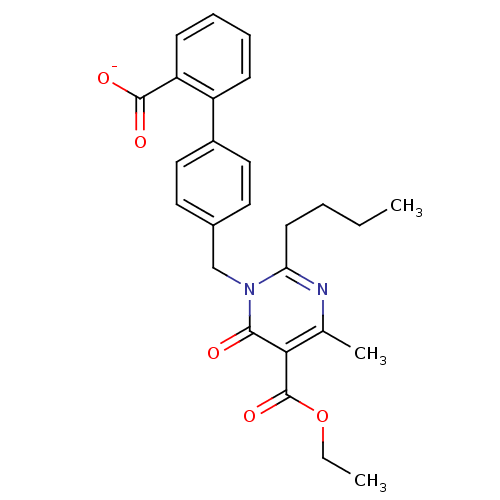 (CHEMBL145276 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004156 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-(4-ch...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Homo sapiens (Human)) | BDBM50004704 ((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of guinea pig striated muscle | Bioorg Med Chem Lett 3: 2797-2800 (1993) Article DOI: 10.1016/S0960-894X(01)80767-8 BindingDB Entry DOI: 10.7270/Q2FN164V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Homo sapiens (Human)) | BDBM50281271 ((2R,3S)-5-(2-Dimethylamino-ethyl)-2-(4-methoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of guinea pig striated muscle | Bioorg Med Chem Lett 3: 2797-2800 (1993) Article DOI: 10.1016/S0960-894X(01)80767-8 BindingDB Entry DOI: 10.7270/Q2FN164V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Homo sapiens (Human)) | BDBM50000383 ((3R,4R)-1-(2-Dimethylamino-ethyl)-4-(4-methoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of guinea pig striated muscle | Bioorg Med Chem Lett 3: 2797-2800 (1993) Article DOI: 10.1016/S0960-894X(01)80767-8 BindingDB Entry DOI: 10.7270/Q2FN164V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004157 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-6-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004158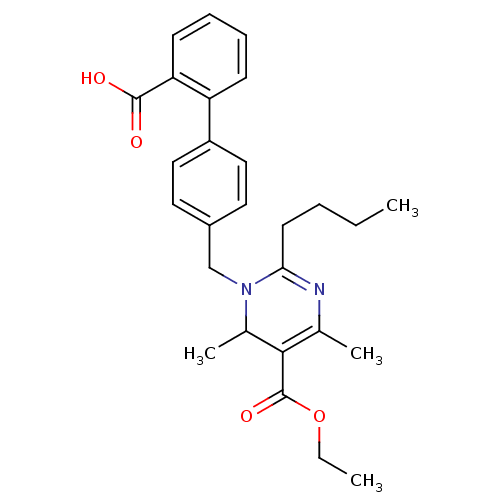 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004159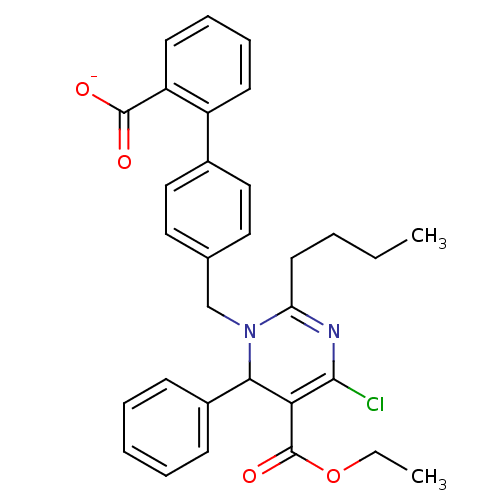 (CHEMBL343622 | Sodium; 4'-(2-butyl-4-chloro-5-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004165 (CHEMBL144952 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5668 (2-amino-5-thio-substituted thiazole 45 | BMS-38703...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5924 (BMS-387032 analog 14 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5660 (2-amino-5-thio-substituted thiazole 25 | 2-aminoth...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5930 (BMS-387032 analog 20 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5934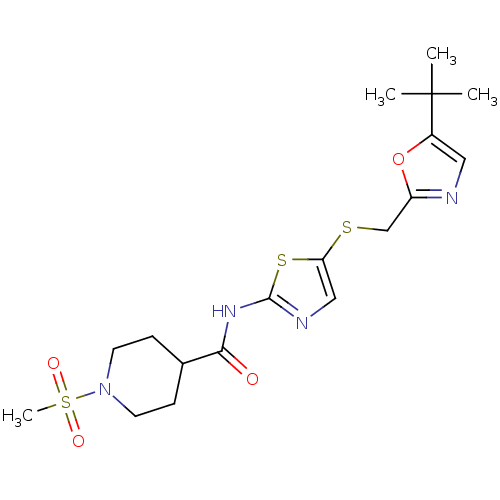 (BMS-387032 analog 24 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5923 (3-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5936 (4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5683 (2-amino-5-thio-substituted thiazole 31 | BMS-38703...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5932 (1-Methyl-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5931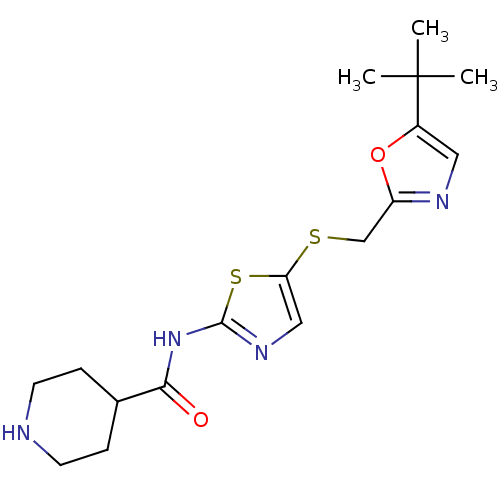 (BMS-387072 | CHEMBL296468 | N-(5-{[(5-tert-butyl-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5926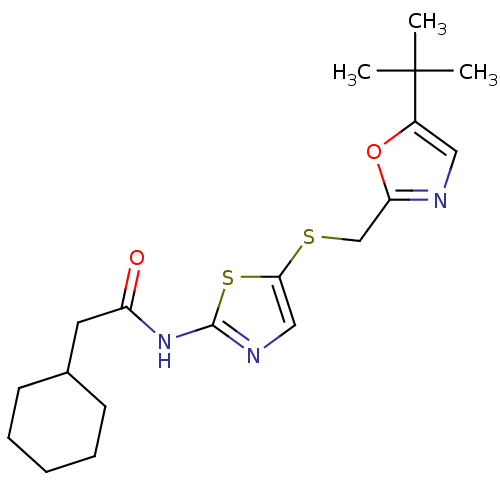 (BMS-387032 analog 16 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5928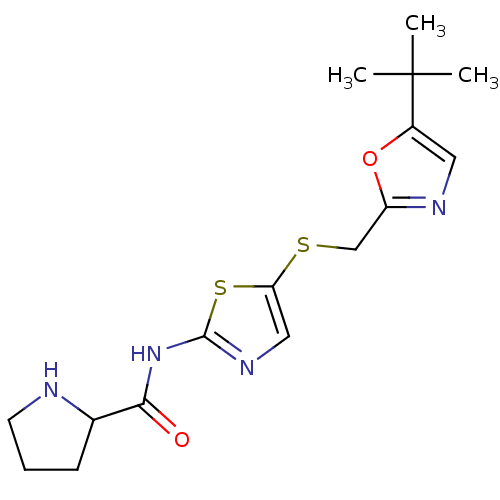 ((+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]met...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5933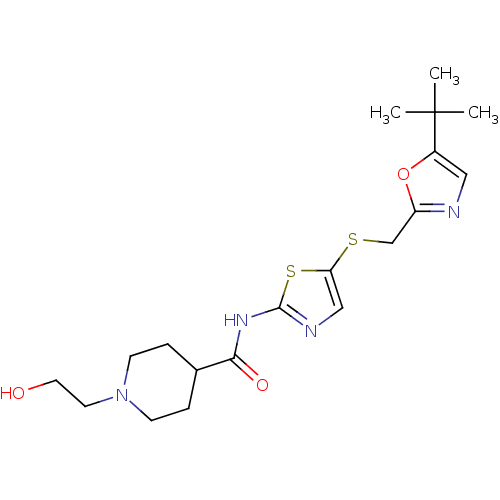 (BMS-387032 analog 23 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5929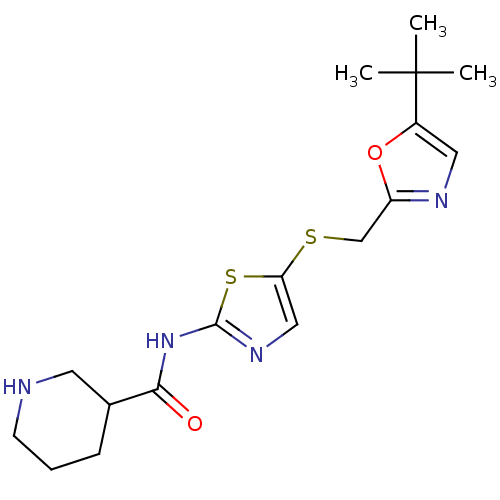 ((+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]met...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5937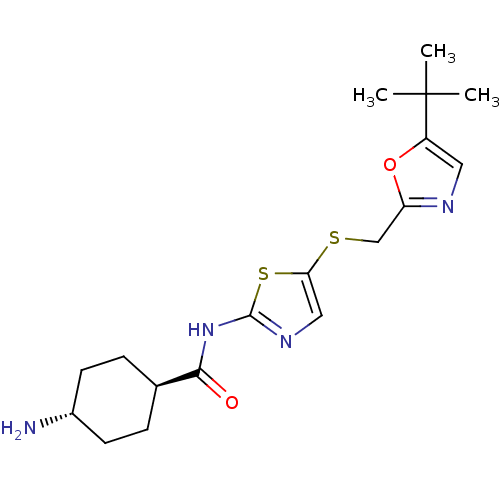 (4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5935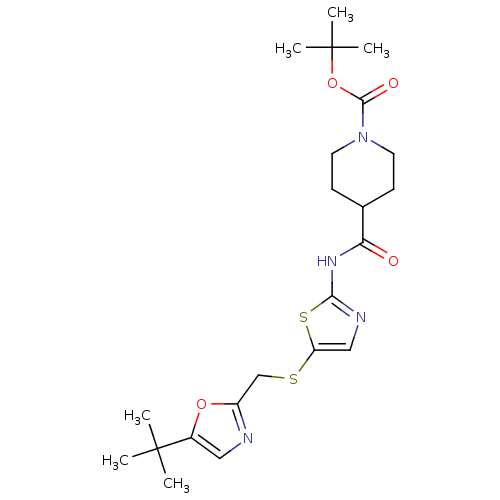 (BMS-387032 analog 25 | N-[5-[[[5-(1,1-Dimethylethy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5927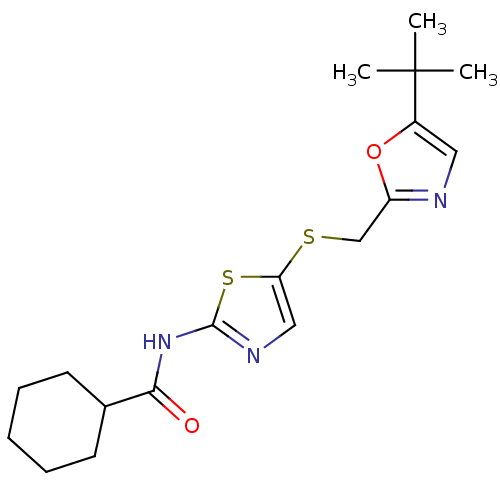 (BMS-387032 analog 17 | N-(5-{[(5-tert-butyl-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 47: 1719-28 (2004) Article DOI: 10.1021/jm0305568 BindingDB Entry DOI: 10.7270/Q26971SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||