

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282171 (CHEMBL4176742) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 15 mins followed by substra... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282177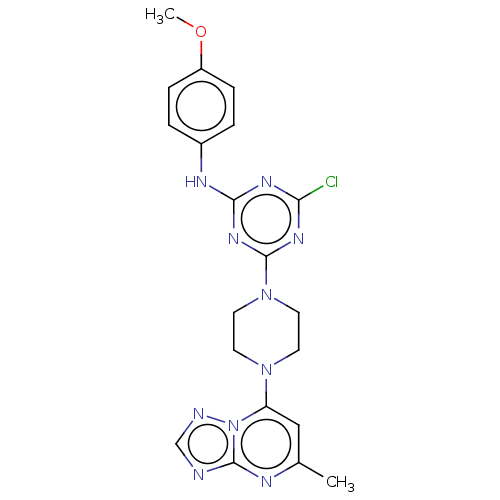 (CHEMBL4172159) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 15 mins followed by substra... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50377588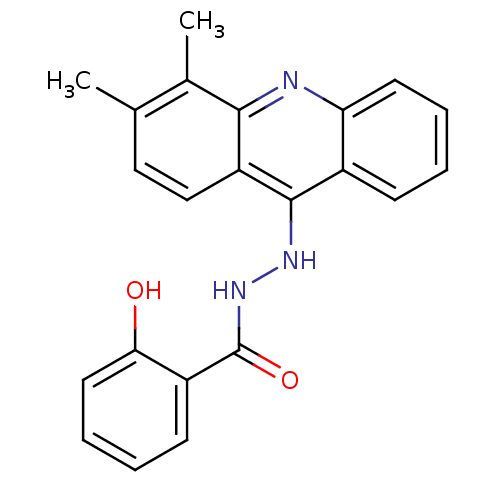 (CHEMBL257594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50377588 (CHEMBL257594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50377587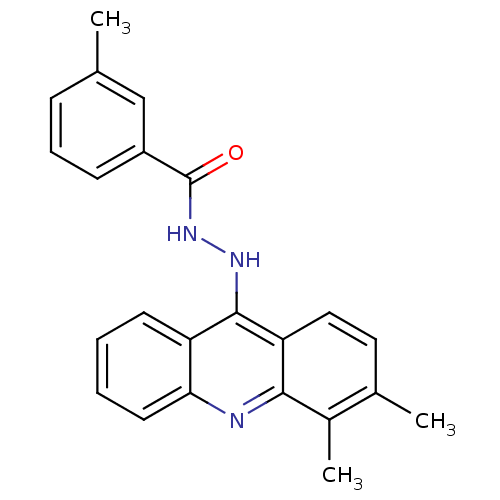 (CHEMBL404394) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50377587 (CHEMBL404394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase (TS) in human | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282171 (CHEMBL4176742) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282177 (CHEMBL4172159) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of GAR transformylase in hog liver | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448067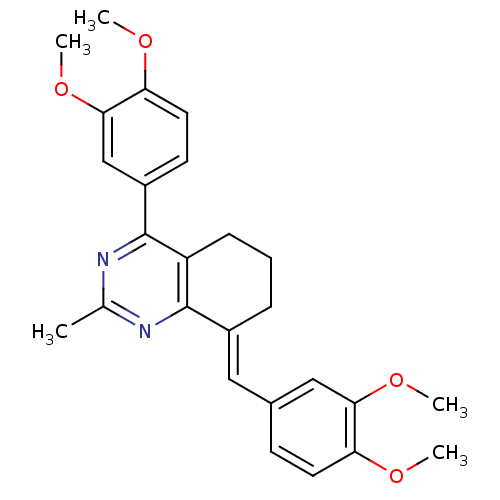 (CHEMBL3115730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282182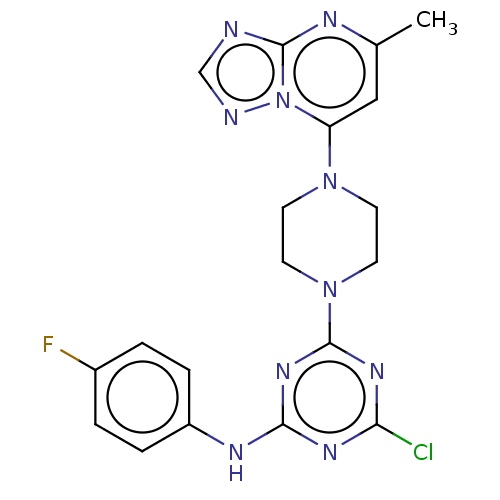 (CHEMBL4161552) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of GAR transformylase in hog liver | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282181 (CHEMBL4168812) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282187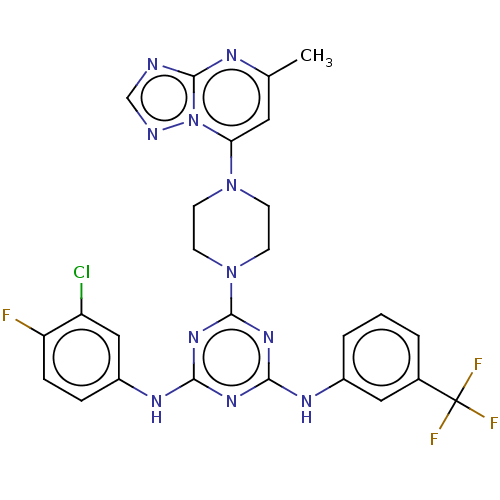 (CHEMBL4173299) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of 5-aminoimidazole-4-carboxamide AICAR formyltransferase in MOLT-4 cells | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282185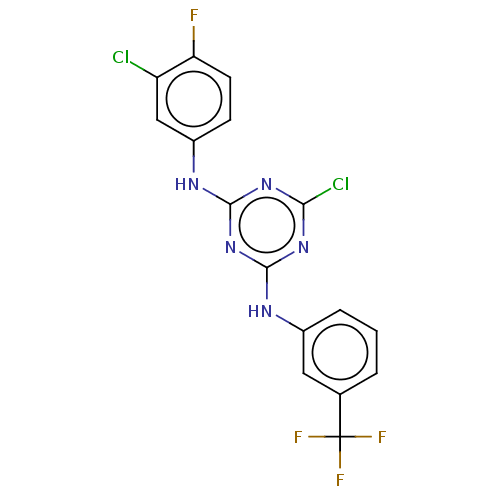 (CHEMBL4177486) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase (DHFR) in WIL2 cells | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282184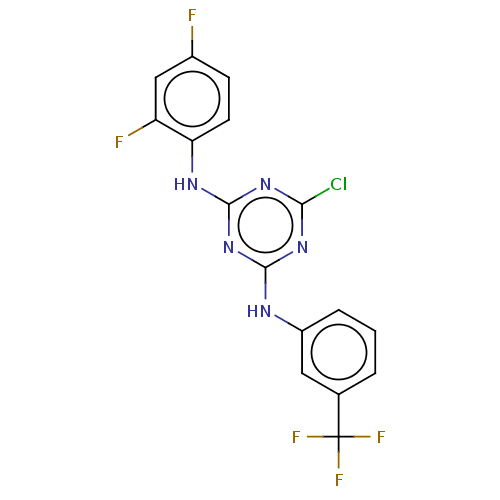 (CHEMBL4175196) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448066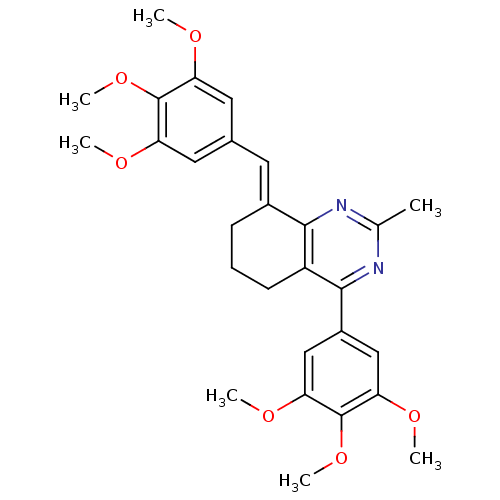 (CHEMBL3115731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282186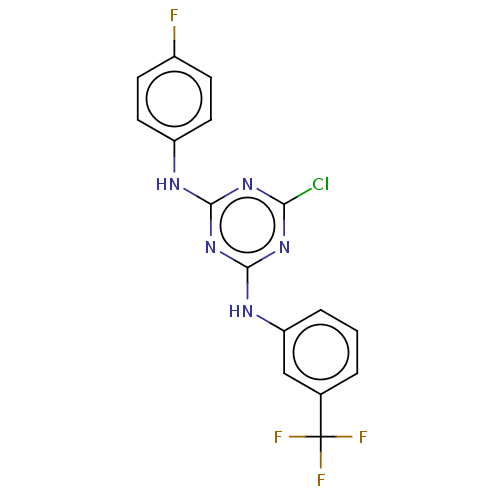 (CHEMBL4170265) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282188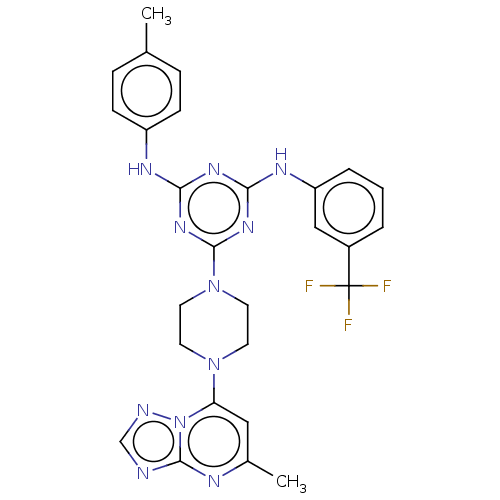 (CHEMBL4165386) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 558 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase (TS) in human | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282180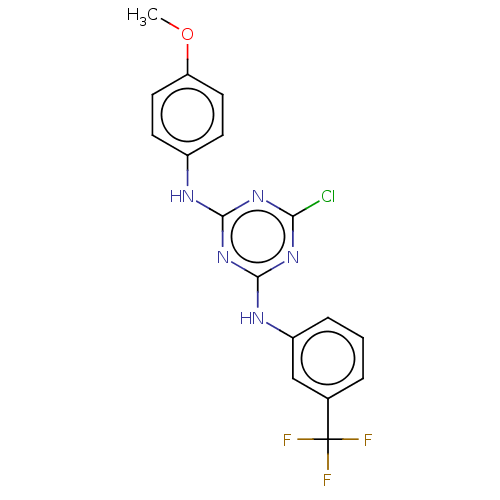 (CHEMBL4166865) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of GAR transformylase in hog liver | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282175 (CHEMBL4162346) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 672 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282178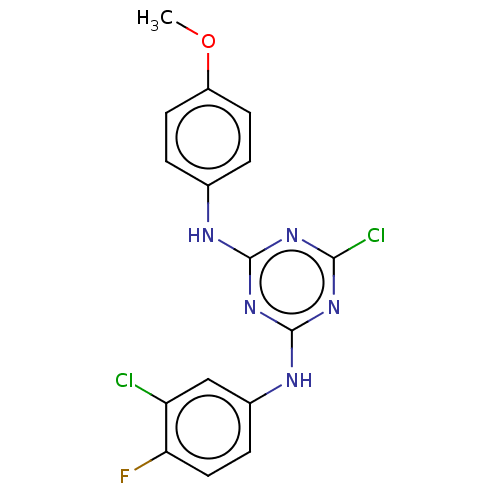 (CHEMBL4174114) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 705 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282172 (CHEMBL4161947) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase (TS) in human | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282176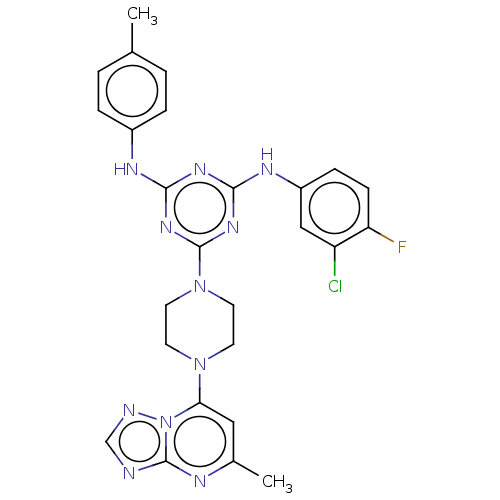 (CHEMBL4169871) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 859 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282173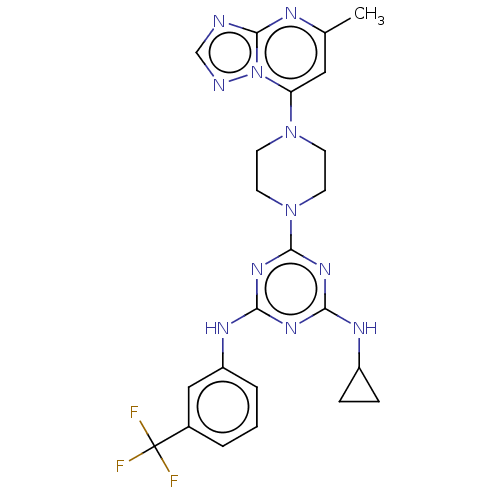 (CHEMBL4176666) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 903 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282174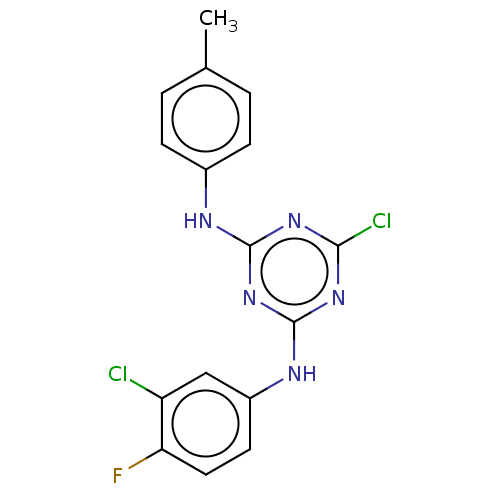 (CHEMBL4163412) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 917 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282182 (CHEMBL4161552) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of GAR transformylase in hog liver | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50062329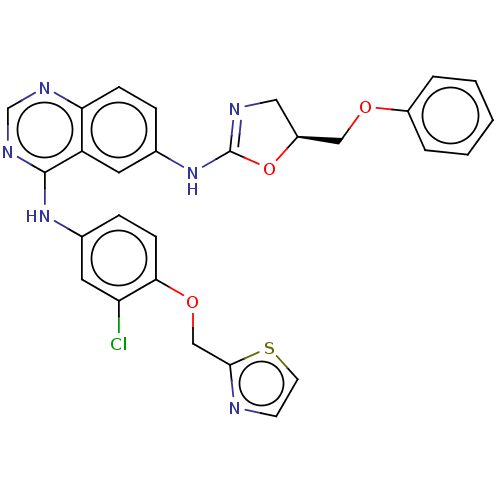 (CHEMBL3397276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM233139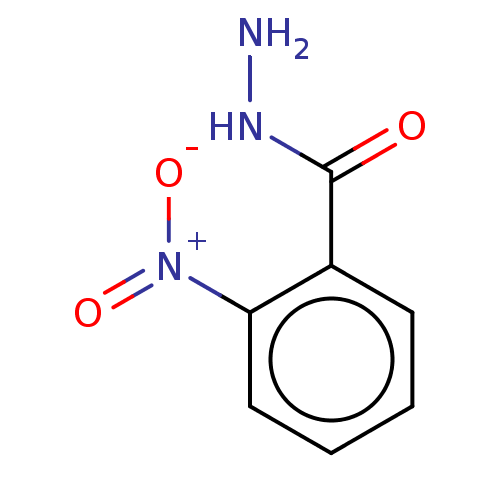 (2-Nitrobenzohydrazide | Mr-I-53b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM233141 (1,2-Diphenylhydrazine | Mr-II-10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50062292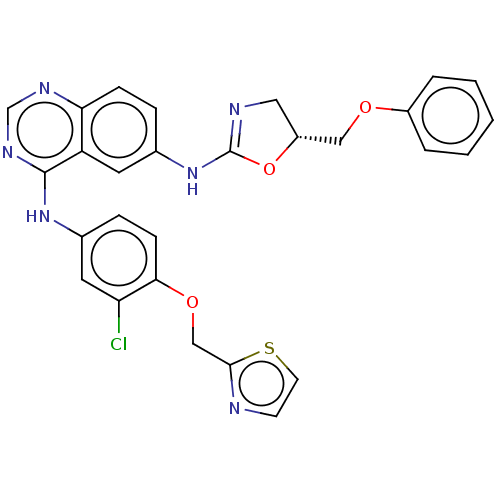 (CHEMBL3397275) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM233138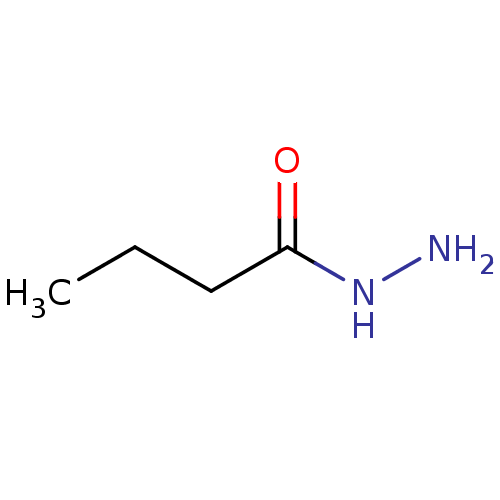 (Butanohydrazide | Mr-I-33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50282183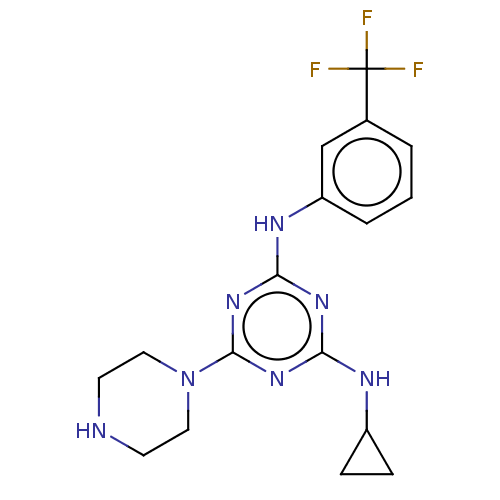 (CHEMBL4167322) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of 5-aminoimidazole-4-carboxamide AICAR formyltransferase in MOLT-4 cells | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM233139 (2-Nitrobenzohydrazide | Mr-I-53b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM233140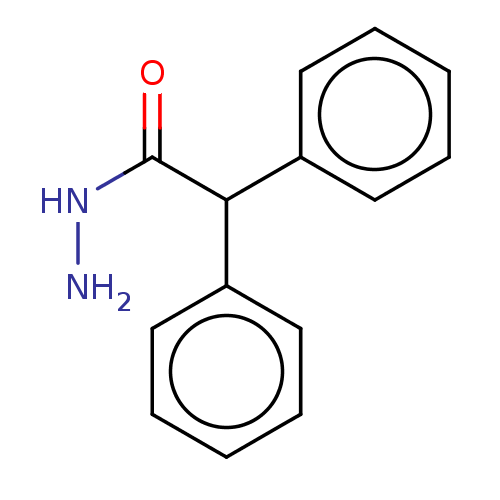 (2-Nitrobenzohydrazide | Mr-I-179) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50565796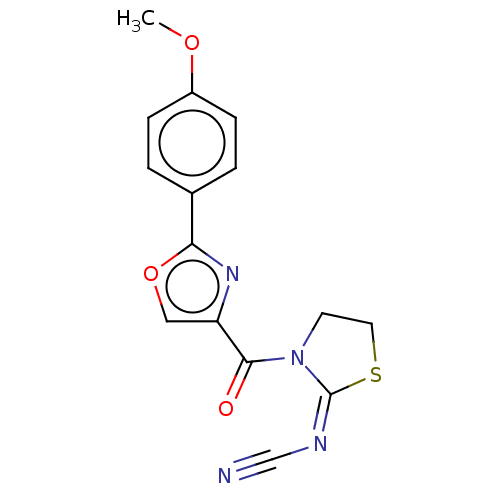 (CHEMBL4780773) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human U-937 cells derived PDE4B using [3H] cAMP as substrate measured after 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112795 BindingDB Entry DOI: 10.7270/Q2MK6HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM233138 (Butanohydrazide | Mr-I-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM233142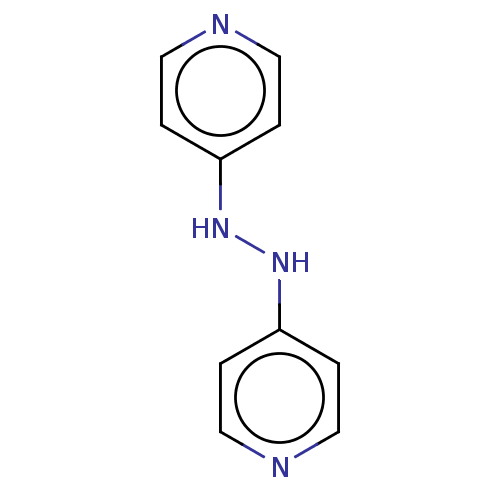 (1,2-Dipyridinylhydrazine | Mr-II-80) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50001676 (CHEMBL3237776 | Mr-I-27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282177 (CHEMBL4172159) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase (TS) in human | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM233140 (2-Nitrobenzohydrazide | Mr-I-179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282178 (CHEMBL4174114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001676 (CHEMBL3237776 | Mr-I-27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of Karachi | Assay Description The assay was performed with plasmepsin-II/cathepsin D (1.2 nM) and substrate (malaria FRET-1; 1.0 μM) in 0.1 M sodium acetate buffer pH 5, contai... | J Enzyme Inhib Med Chem 25: 673-8 (2010) Article DOI: 10.3109/14756360903508430 BindingDB Entry DOI: 10.7270/Q29885WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282188 (CHEMBL4165386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase (DHFR) in WIL2 cells | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282171 (CHEMBL4176742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50282173 (CHEMBL4176666) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia (Central University) Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Eur J Med Chem 136: 36-51 (2017) Article DOI: 10.1016/j.ejmech.2017.04.064 BindingDB Entry DOI: 10.7270/Q2G73H9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 166 total ) | Next | Last >> |