

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50559204 (CHEMBL4753756) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human XIAP-BIR3 (241 to 356 residues) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127676 BindingDB Entry DOI: 10.7270/Q2NV9NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588041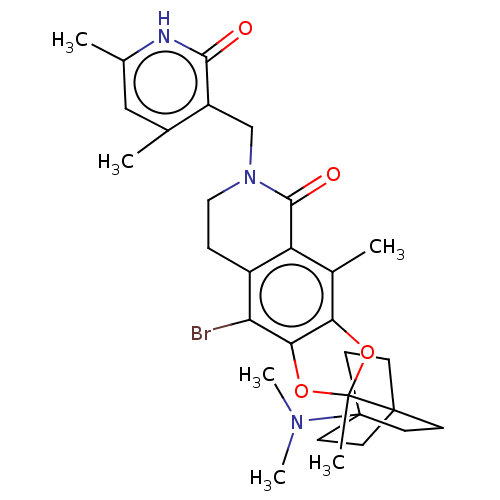 (9-bromo-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588031 (9-chloro-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731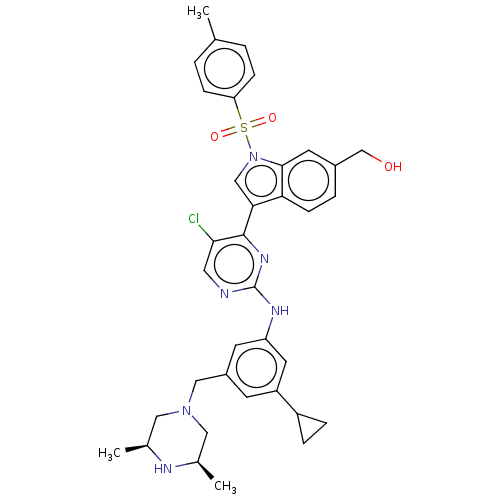 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588042 (6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-yl)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM185584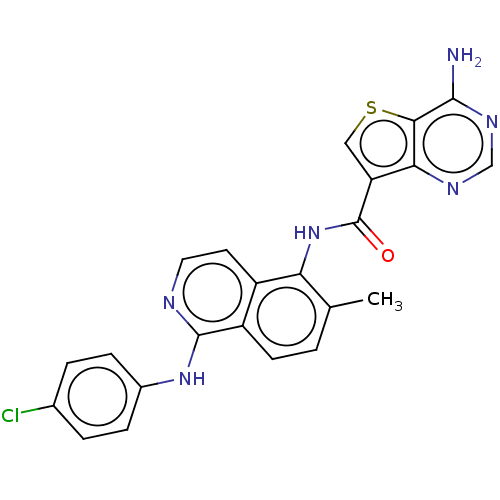 (US9156852, 1 | USRE47451, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic... | US Patent US9156852 (2015) BindingDB Entry DOI: 10.7270/Q24T6H4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM185584 (US9156852, 1 | USRE47451, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2XW4N61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700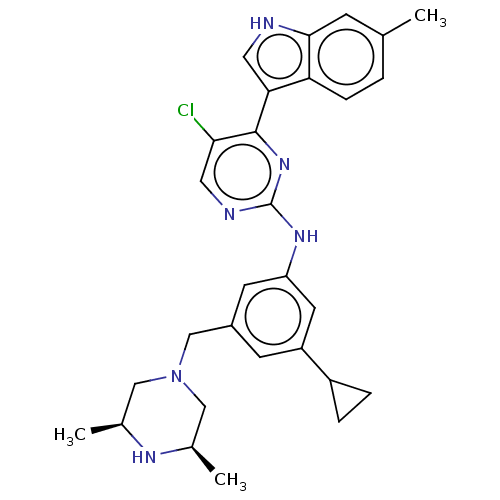 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588032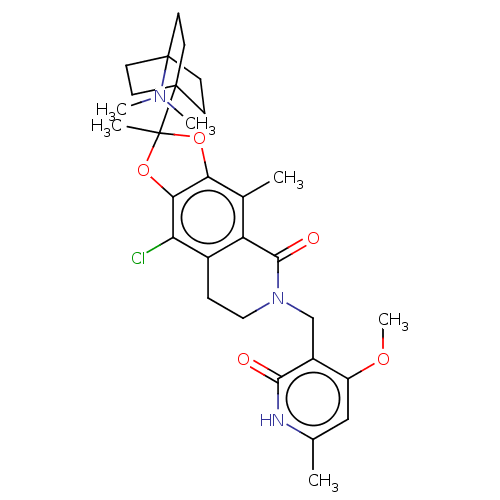 (9-chloro-2-(4- (dimethylamino)bicyclo[2.2.2]octan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588043 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588052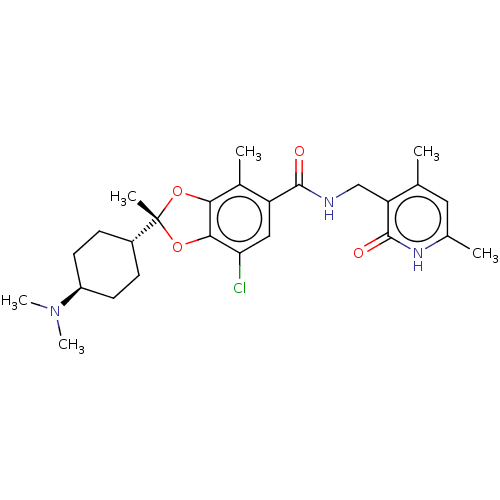 ((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE UniChem | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695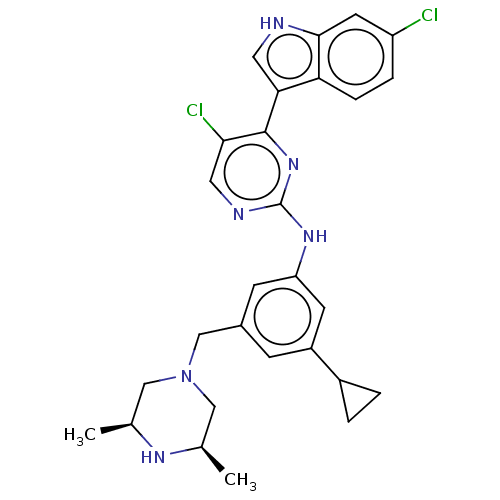 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711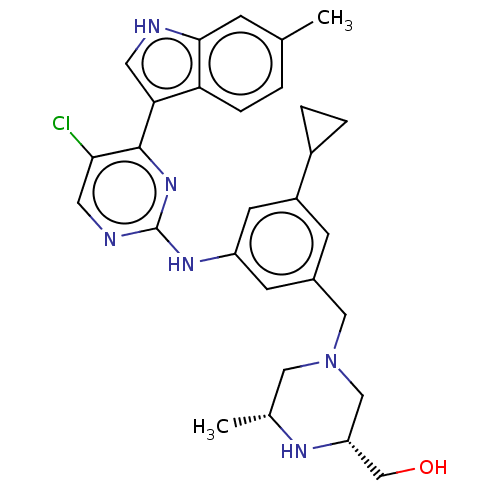 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476699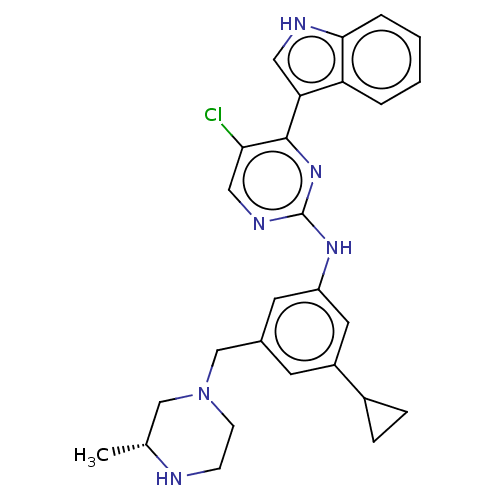 ((R)-5-chloro-N-(3-cyclopropyl-5-((3-methylpiperazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476699 ((R)-5-chloro-N-(3-cyclopropyl-5-((3-methylpiperazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM185584 (US9156852, 1 | USRE47451, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic... | US Patent US9156852 (2015) BindingDB Entry DOI: 10.7270/Q24T6H4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epithelial discoidin domain-containing receptor 1 (Homo sapiens (Human)) | BDBM185584 (US9156852, 1 | USRE47451, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2XW4N61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588046 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476710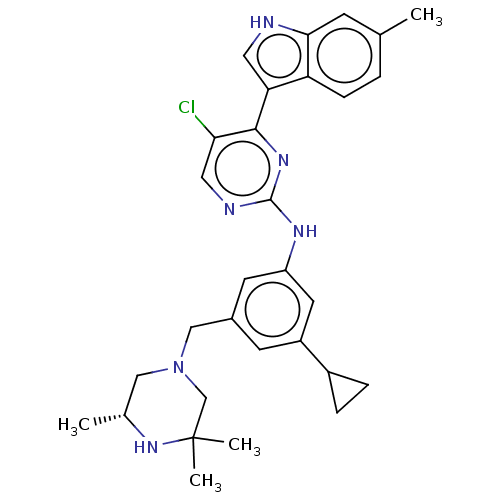 ((R)-5-chloro-N-(3-cyclopropyl-5-((3,3,5-trimethylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476710 ((R)-5-chloro-N-(3-cyclopropyl-5-((3,3,5-trimethylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476708 (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476708 (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038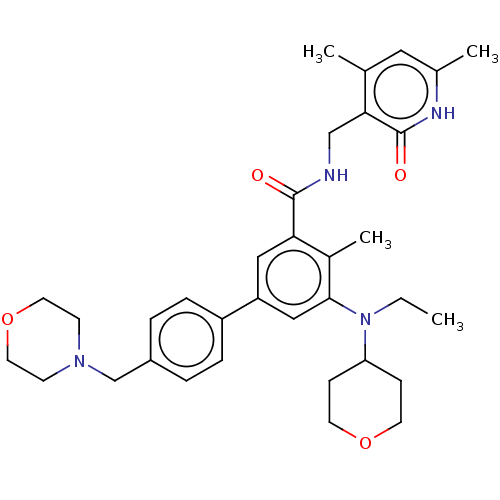 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588044 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588050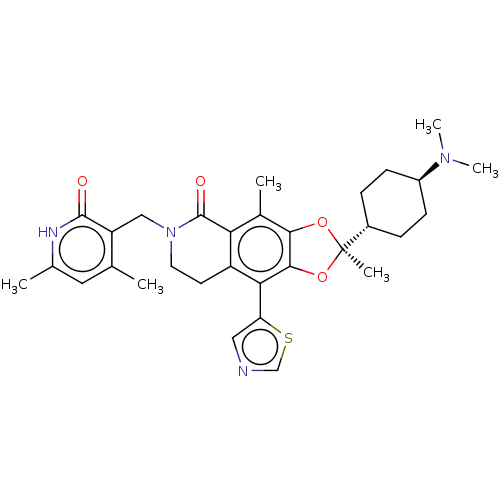 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238347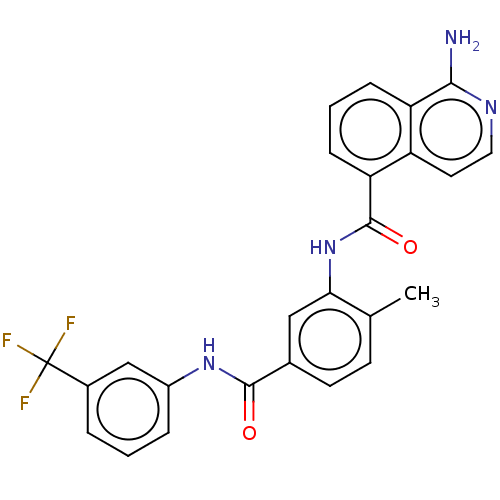 (US9388165, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588048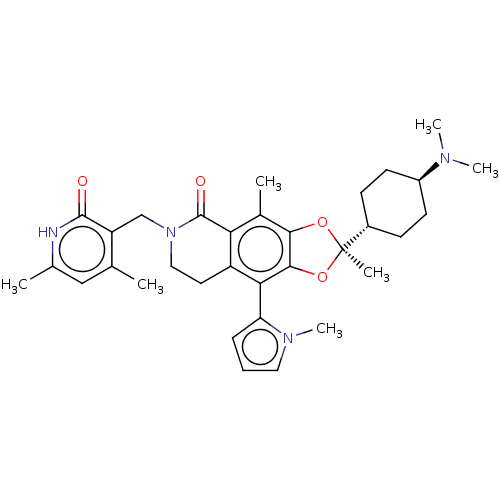 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588047 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM588045 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476701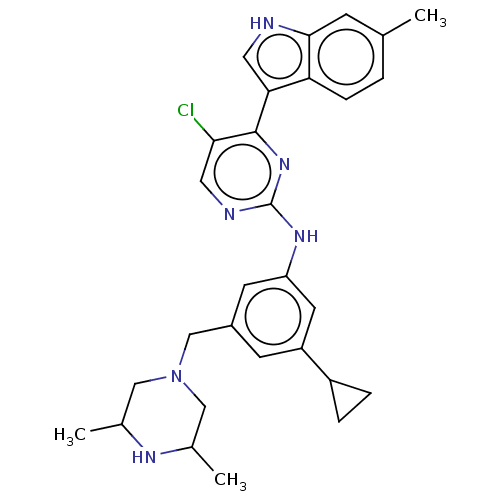 (US10870639, Example 10 | US11292786, Example 10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476701 (US10870639, Example 10 | US11292786, Example 10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476697 (2-((2R,6S)-4-(3-((5-chloro-4-(1H-indole-3-yl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 320 total ) | Next | Last >> |