 Found 1253 hits with Last Name = 'ahuja' and Initial = 'v'
Found 1253 hits with Last Name = 'ahuja' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50395823

(CHEMBL2165205)Show SMILES COCCN(CCOC)Cc1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(25.82,-9.73,;27.14,-10.52,;27.12,-12.06,;25.77,-12.81,;25.76,-14.34,;27.09,-15.12,;28.43,-14.35,;29.76,-15.12,;31.1,-14.36,;24.43,-15.12,;24.44,-16.66,;23.11,-17.43,;23.11,-18.97,;21.78,-19.74,;24.44,-19.74,;25.78,-18.97,;27.25,-19.46,;28.17,-18.2,;29.7,-18.19,;27.25,-16.94,;25.78,-17.42,;27.99,-20.8,;27.19,-22.11,;27.92,-23.47,;29.46,-23.5,;30.21,-24.85,;30.26,-22.17,;29.52,-20.83,;30.32,-19.51,)| Show InChI InChI=1S/C21H26Cl2N4O2/c1-14-11-17(13-26(7-9-28-3)8-10-29-4)27-21(24-14)20(15(2)25-27)18-6-5-16(22)12-19(18)23/h5-6,11-12H,7-10,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Binding affinity to CRFR1 by autoradiography |
Bioorg Med Chem Lett 22: 6651-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.112
BindingDB Entry DOI: 10.7270/Q20C4WWB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50152162

(CHEMBL188844 | CHEMBL2165204 | [3-(6-Dimethylamino...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1ccc(nc1)N(C)C Show InChI InChI=1S/C21H30N6/c1-7-11-26(12-8-2)19-13-15(3)23-21-20(16(4)24-27(19)21)17-9-10-18(22-14-17)25(5)6/h9-10,13-14H,7-8,11-12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Binding affinity to CRFR1 by autoradiography |
Bioorg Med Chem Lett 22: 6651-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.112
BindingDB Entry DOI: 10.7270/Q20C4WWB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM86192

(CAS_197801-88-0 | CHEMBL2165203 | SN003)Show InChI InChI=1S/C19H25N5O2/c1-6-14(11-25-4)24-17-10-13(3)20-19(18(17)22-23-24)21-16-8-7-15(26-5)9-12(16)2/h7-10,14H,6,11H2,1-5H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Binding affinity to CRFR1 by autoradiography |
Bioorg Med Chem Lett 22: 6651-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.112
BindingDB Entry DOI: 10.7270/Q20C4WWB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50395822
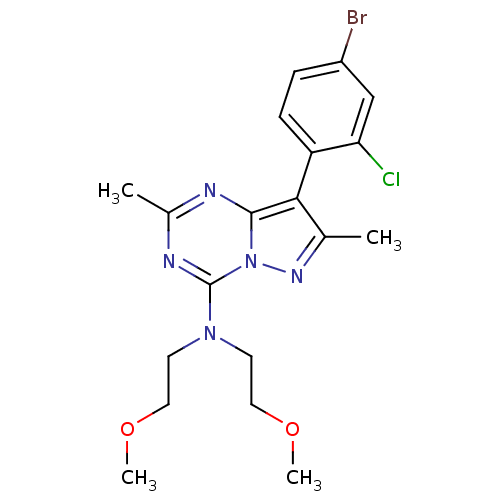
(CHEMBL1762817)Show SMILES COCCN(CCOC)c1nc(C)nc2c(c(C)nn12)-c1ccc(Br)cc1Cl |(-.62,5.04,;-1.96,5.81,;-3.29,5.03,;-4.63,5.8,;-5.96,5.03,;-7.29,5.79,;-8.63,5.02,;-9.96,5.78,;-11.29,5.01,;-5.95,3.49,;-7.28,2.71,;-7.28,1.17,;-8.62,.4,;-5.95,.4,;-4.62,1.17,;-3.14,.69,;-2.23,1.95,;-.69,1.95,;-3.14,3.2,;-4.62,2.72,;-2.34,-.61,;-3.16,-1.92,;-2.44,-3.28,;-.9,-3.34,;-.17,-4.69,;-.08,-2.02,;-.8,-.67,;.01,.64,)| Show InChI InChI=1S/C19H23BrClN5O2/c1-12-17(15-6-5-14(20)11-16(15)21)18-22-13(2)23-19(26(18)24-12)25(7-9-27-3)8-10-28-4/h5-6,11H,7-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Binding affinity to CRFR1 by autoradiography |
Bioorg Med Chem Lett 22: 6651-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.112
BindingDB Entry DOI: 10.7270/Q20C4WWB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50395822

(CHEMBL1762817)Show SMILES COCCN(CCOC)c1nc(C)nc2c(c(C)nn12)-c1ccc(Br)cc1Cl |(-.62,5.04,;-1.96,5.81,;-3.29,5.03,;-4.63,5.8,;-5.96,5.03,;-7.29,5.79,;-8.63,5.02,;-9.96,5.78,;-11.29,5.01,;-5.95,3.49,;-7.28,2.71,;-7.28,1.17,;-8.62,.4,;-5.95,.4,;-4.62,1.17,;-3.14,.69,;-2.23,1.95,;-.69,1.95,;-3.14,3.2,;-4.62,2.72,;-2.34,-.61,;-3.16,-1.92,;-2.44,-3.28,;-.9,-3.34,;-.17,-4.69,;-.08,-2.02,;-.8,-.67,;.01,.64,)| Show InChI InChI=1S/C19H23BrClN5O2/c1-12-17(15-6-5-14(20)11-16(15)21)18-22-13(2)23-19(26(18)24-12)25(7-9-27-3)8-10-28-4/h5-6,11H,7-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to CRF1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 2052-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.009
BindingDB Entry DOI: 10.7270/Q2DR2WW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610453
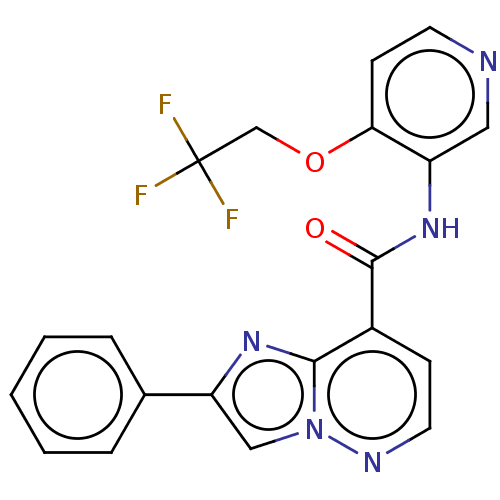
(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463
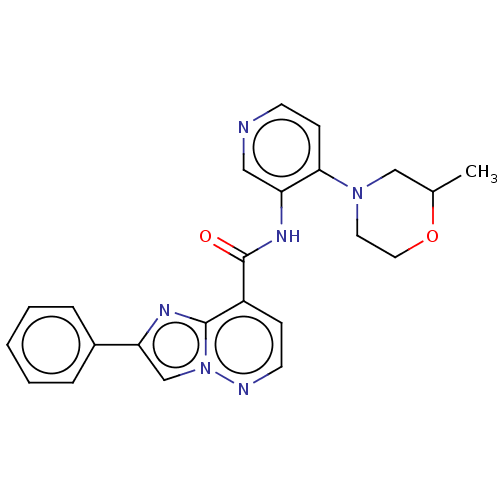
(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610459

(N-(4-(4-Cyanopiperidin-1-yl)pyridin-3-yl)-2-phenyl...)Show SMILES O=C(Nc1cnccc1N1CCC(CC1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610453

(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610486

(2-(Cyclohex-1-en-1-yl)-N-(4-(4,4-difluoropiperidin...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1=CCCCC1 |t:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | CHEMBL5266553

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610483

(2-Cyclopropyl-N-(4-(4,4-difluoropiperidin-1-yl)pyr...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610460
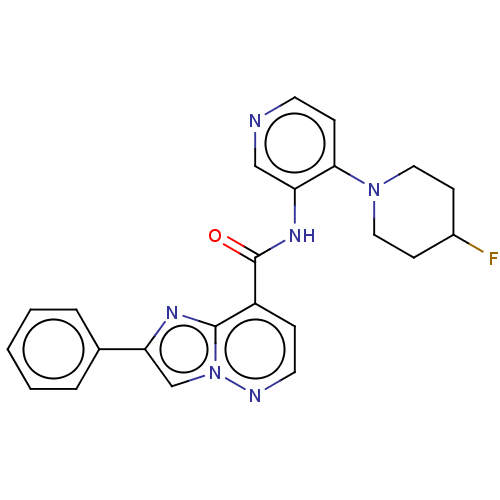
(N-(4-(4-Fluoropiperidin-1-yl)pyridin-3-yl)-2-pheny...)Show SMILES FC1CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610461

(N-(4-(4,4-Difluoropiperidin-1-yl)pyridin-3-yl)-2-p...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610462
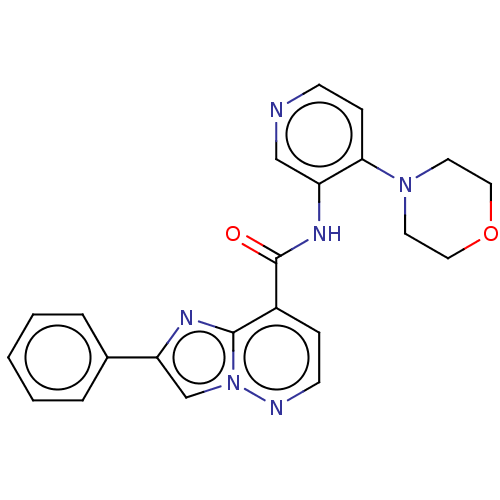
(N-(4-Morpholinopyridin-3-yl)-2-phenylimidazo[1,2-b...)Show SMILES O=C(Nc1cnccc1N1CCOCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610458
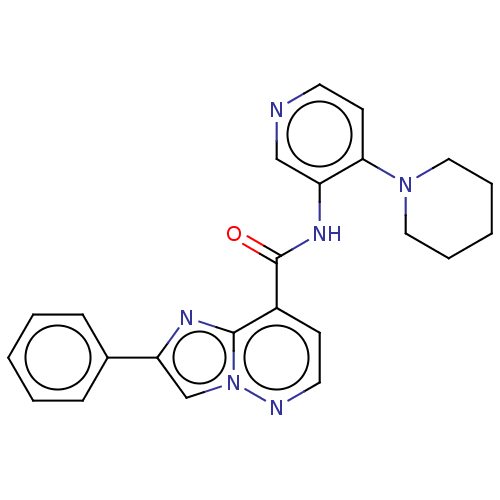
(2-Phenyl-N-(4-(piperidin-1-yl)pyridin-3-yl)imidazo...)Show SMILES O=C(Nc1cnccc1N1CCCCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610477
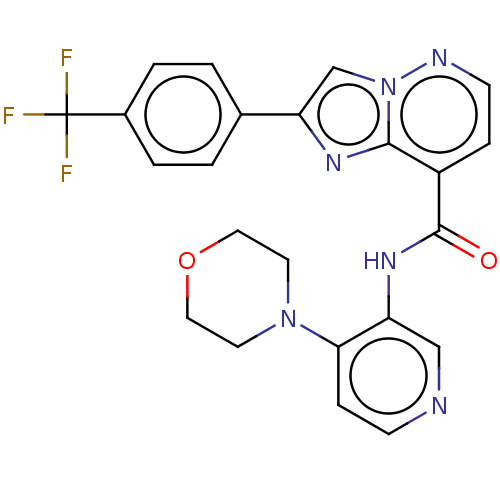
(N-(4-Morpholinopyridin-3-yl)-2-(4-(trifluoromethyl...)Show SMILES FC(F)(F)c1ccc(cc1)-c1cn2nccc(C(=O)Nc3cnccc3N3CCOCC3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610459

(N-(4-(4-Cyanopiperidin-1-yl)pyridin-3-yl)-2-phenyl...)Show SMILES O=C(Nc1cnccc1N1CCC(CC1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610483

(2-Cyclopropyl-N-(4-(4,4-difluoropiperidin-1-yl)pyr...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610473
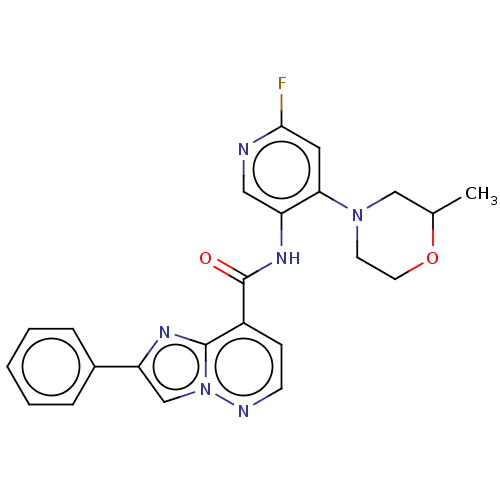
(N-(6-Fluoro-4-morpholinopyridin-3-yl)-2-phenylimid...)Show SMILES CC1CN(CCO1)c1cc(F)ncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610462

(N-(4-Morpholinopyridin-3-yl)-2-phenylimidazo[1,2-b...)Show SMILES O=C(Nc1cnccc1N1CCOCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300149
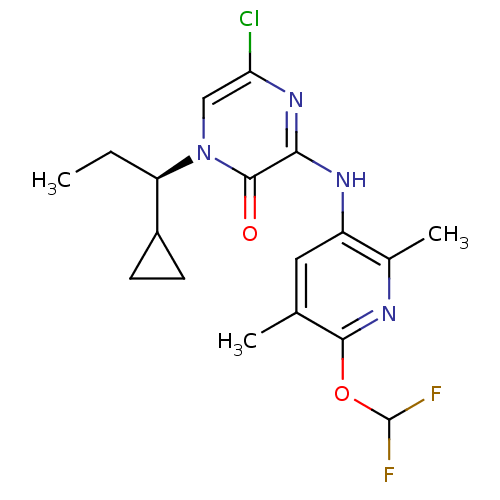
((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(6-(difluor...)Show SMILES CC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C18H21ClF2N4O2/c1-4-13(11-5-6-11)25-8-14(19)24-15(17(25)26)23-12-7-9(2)16(22-10(12)3)27-18(20)21/h7-8,11,13,18H,4-6H2,1-3H3,(H,23,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay |
J Med Chem 52: 7653-68 (2009)
Article DOI: 10.1021/jm900716v
BindingDB Entry DOI: 10.7270/Q2CN740W |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610461

(N-(4-(4,4-Difluoropiperidin-1-yl)pyridin-3-yl)-2-p...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610477

(N-(4-Morpholinopyridin-3-yl)-2-(4-(trifluoromethyl...)Show SMILES FC(F)(F)c1ccc(cc1)-c1cn2nccc(C(=O)Nc3cnccc3N3CCOCC3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300145
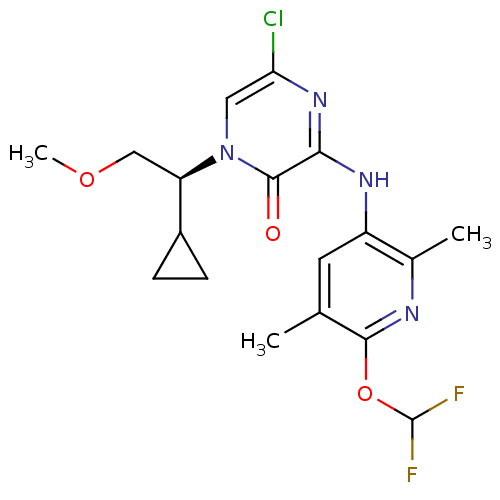
((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...)Show SMILES COC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C18H21ClF2N4O3/c1-9-6-12(10(2)22-16(9)28-18(20)21)23-15-17(26)25(7-14(19)24-15)13(8-27-3)11-4-5-11/h6-7,11,13,18H,4-5,8H2,1-3H3,(H,23,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay |
J Med Chem 52: 7653-68 (2009)
Article DOI: 10.1021/jm900716v
BindingDB Entry DOI: 10.7270/Q2CN740W |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610458

(2-Phenyl-N-(4-(piperidin-1-yl)pyridin-3-yl)imidazo...)Show SMILES O=C(Nc1cnccc1N1CCCCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300145

((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...)Show SMILES COC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C18H21ClF2N4O3/c1-9-6-12(10(2)22-16(9)28-18(20)21)23-15-17(26)25(7-14(19)24-15)13(8-27-3)11-4-5-11/h6-7,11,13,18H,4-5,8H2,1-3H3,(H,23,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting |
Bioorg Med Chem Lett 20: 1890-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.129
BindingDB Entry DOI: 10.7270/Q29G5MZ4 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50293912
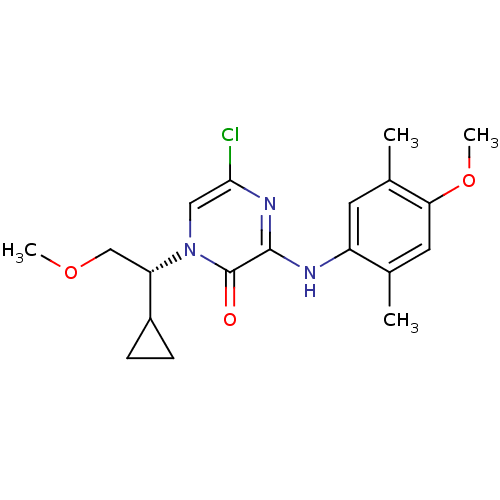
((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...)Show SMILES COC[C@@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)cc2C)c1=O |r| Show InChI InChI=1S/C19H24ClN3O3/c1-11-8-16(26-4)12(2)7-14(11)21-18-19(24)23(9-17(20)22-18)15(10-25-3)13-5-6-13/h7-9,13,15H,5-6,10H2,1-4H3,(H,21,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-o-CRF from CRFR1 in rat frontal cortex after 2 hrs by gamma counter |
Bioorg Med Chem Lett 22: 6651-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.112
BindingDB Entry DOI: 10.7270/Q20C4WWB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50293912

((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...)Show SMILES COC[C@@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)cc2C)c1=O |r| Show InChI InChI=1S/C19H24ClN3O3/c1-11-8-16(26-4)12(2)7-14(11)21-18-19(24)23(9-17(20)22-18)15(10-25-3)13-5-6-13/h7-9,13,15H,5-6,10H2,1-4H3,(H,21,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting |
J Med Chem 52: 4173-91 (2009)
Article DOI: 10.1021/jm900301y
BindingDB Entry DOI: 10.7270/Q2G44Q9K |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50293912

((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...)Show SMILES COC[C@@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)cc2C)c1=O |r| Show InChI InChI=1S/C19H24ClN3O3/c1-11-8-16(26-4)12(2)7-14(11)21-18-19(24)23(9-17(20)22-18)15(10-25-3)13-5-6-13/h7-9,13,15H,5-6,10H2,1-4H3,(H,21,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique |
J Med Chem 52: 4161-72 (2009)
Article DOI: 10.1021/jm900302q
BindingDB Entry DOI: 10.7270/Q2WW7JM0 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50293974
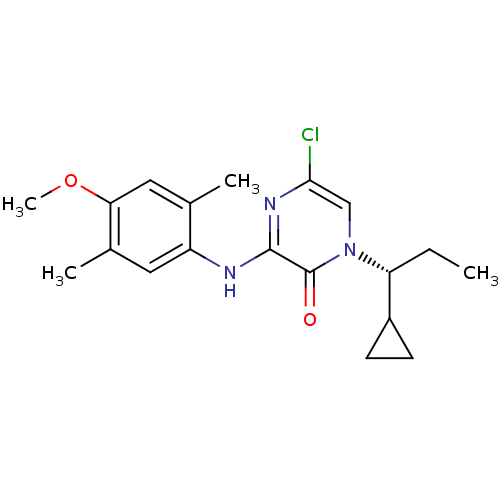
((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...)Show SMILES CC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)cc2C)c1=O |r| Show InChI InChI=1S/C19H24ClN3O2/c1-5-15(13-6-7-13)23-10-17(20)22-18(19(23)24)21-14-8-12(3)16(25-4)9-11(14)2/h8-10,13,15H,5-7H2,1-4H3,(H,21,22)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting |
J Med Chem 52: 4173-91 (2009)
Article DOI: 10.1021/jm900301y
BindingDB Entry DOI: 10.7270/Q2G44Q9K |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300136
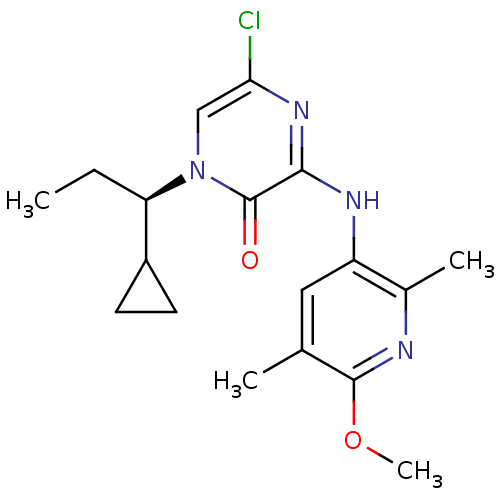
((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(6-methoxy-...)Show SMILES CC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)nc2C)c1=O |r| Show InChI InChI=1S/C18H23ClN4O2/c1-5-14(12-6-7-12)23-9-15(19)22-16(18(23)24)21-13-8-10(2)17(25-4)20-11(13)3/h8-9,12,14H,5-7H2,1-4H3,(H,21,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay |
J Med Chem 52: 7653-68 (2009)
Article DOI: 10.1021/jm900716v
BindingDB Entry DOI: 10.7270/Q2CN740W |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50293964
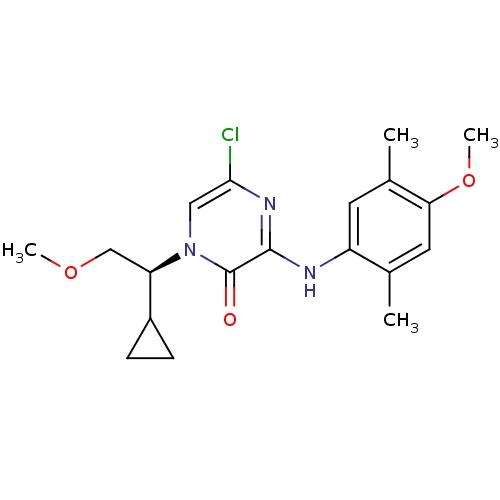
((S)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...)Show SMILES COC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC)cc2C)c1=O |r| Show InChI InChI=1S/C19H24ClN3O3/c1-11-8-16(26-4)12(2)7-14(11)21-18-19(24)23(9-17(20)22-18)15(10-25-3)13-5-6-13/h7-9,13,15H,5-6,10H2,1-4H3,(H,21,22)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting |
J Med Chem 52: 4173-91 (2009)
Article DOI: 10.1021/jm900301y
BindingDB Entry DOI: 10.7270/Q2G44Q9K |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300145

((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...)Show SMILES COC[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C18H21ClF2N4O3/c1-9-6-12(10(2)22-16(9)28-18(20)21)23-15-17(26)25(7-14(19)24-15)13(8-27-3)11-4-5-11/h6-7,11,13,18H,4-5,8H2,1-3H3,(H,23,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting analysis |
Bioorg Med Chem Lett 26: 2184-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.067
BindingDB Entry DOI: 10.7270/Q2KK9FVR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610469

((S)óN-(4-(3-Methylmorpholino)pyridin-3-yl)-2-pheny...)Show SMILES C[C@H]1COCCN1c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610469

((S)óN-(4-(3-Methylmorpholino)pyridin-3-yl)-2-pheny...)Show SMILES C[C@H]1COCCN1c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610460

(N-(4-(4-Fluoropiperidin-1-yl)pyridin-3-yl)-2-pheny...)Show SMILES FC1CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610457

(N-(4-(4-Cyanophenyl)pyridin-3-yl)-2-phenylimidazo[...)Show SMILES O=C(Nc1cnccc1-c1ccc(cc1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300142

((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[6-(difluoro...)Show SMILES C[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C17H19ClF2N4O2/c1-8-6-12(9(2)21-15(8)26-17(19)20)22-14-16(25)24(7-13(18)23-14)10(3)11-4-5-11/h6-7,10-11,17H,4-5H2,1-3H3,(H,22,23)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay |
J Med Chem 52: 7653-68 (2009)
Article DOI: 10.1021/jm900716v
BindingDB Entry DOI: 10.7270/Q2CN740W |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50300142

((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[6-(difluoro...)Show SMILES C[C@H](C1CC1)n1cc(Cl)nc(Nc2cc(C)c(OC(F)F)nc2C)c1=O |r| Show InChI InChI=1S/C17H19ClF2N4O2/c1-8-6-12(9(2)21-15(8)26-17(19)20)22-14-16(25)24(7-13(18)23-14)10(3)11-4-5-11/h6-7,10-11,17H,4-5H2,1-3H3,(H,22,23)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting |
Bioorg Med Chem Lett 20: 1890-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.129
BindingDB Entry DOI: 10.7270/Q29G5MZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM628983

(N-{4-[(2-{[5-(Dimethylcarbamoyl)-6-(4-methanesulfo...)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3ccnc(Nc4ccc(C(=O)N(C)C)c(n4)N4CCN(CC4)S(C)(=O)=O)c3)c(F)c2)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610453

(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610469

((S)óN-(4-(3-Methylmorpholino)pyridin-3-yl)-2-pheny...)Show SMILES C[C@H]1COCCN1c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | CHEMBL5316226
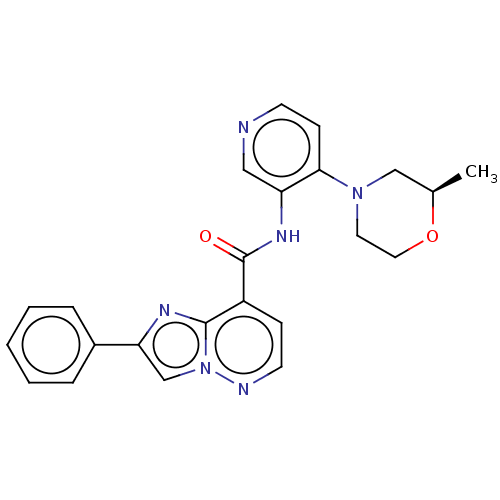
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610476
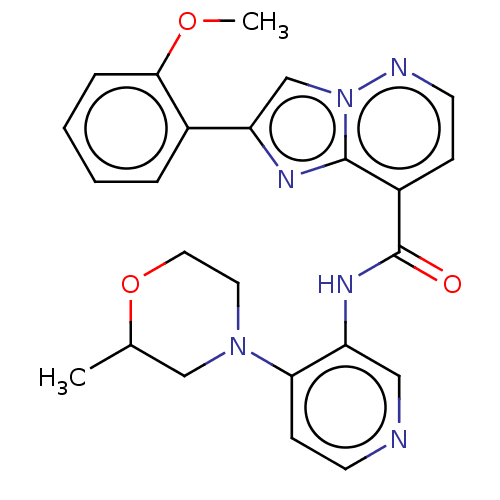
(2-(2-Methoxyphenyl)-N-(4-(2-methylmorpholino)pyrid...)Show SMILES COc1ccccc1-c1cn2nccc(C(=O)Nc3cnccc3N3CCOC(C)C3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610454

(N-(4-(Methylsulfonyl)pyridin-3-yl)-2-phenylimidazo...)Show SMILES CS(=O)(=O)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610488
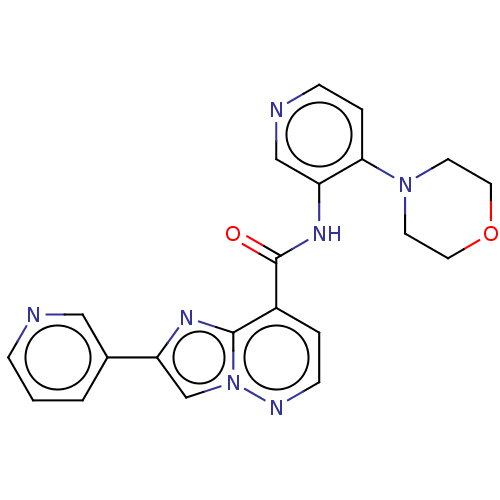
(N-(4-Morpholinopyridin-3-yl)-2-(pyridin-3-yl)imida...)Show SMILES O=C(Nc1cnccc1N1CCOCC1)c1ccnn2cc(nc12)-c1cccnc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data