 Found 134 hits with Last Name = 'aikawa' and Initial = 'm'
Found 134 hits with Last Name = 'aikawa' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500931
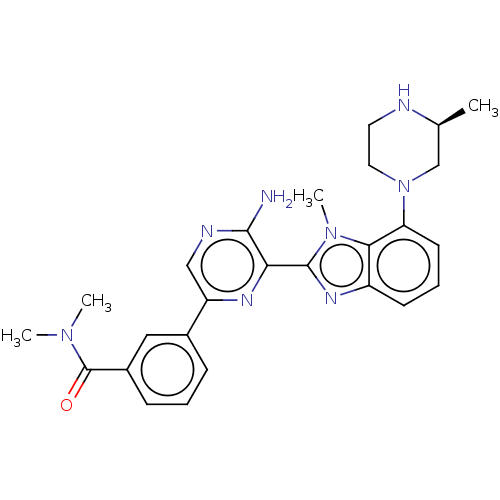
(CHEMBL3797873)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cccc(c3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C26H30N8O/c1-16-15-34(12-11-28-16)21-10-6-9-19-23(21)33(4)25(31-19)22-24(27)29-14-20(30-22)17-7-5-8-18(13-17)26(35)32(2)3/h5-10,13-14,16,28H,11-12,15H2,1-4H3,(H2,27,29)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344541

(4-(2-(4-ethylphenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3o2)cc1 Show InChI InChI=1S/C22H20N4O3/c1-3-14-4-6-15(7-5-14)25-22-26-18-12-16(8-9-20(18)29-22)28-17-10-11-24-19(13-17)21(27)23-2/h4-13H,3H2,1-2H3,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344540
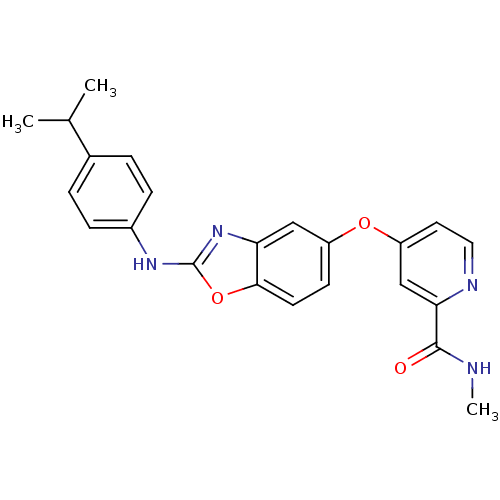
(4-(2-(4-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(cc4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O3/c1-14(2)15-4-6-16(7-5-15)26-23-27-19-12-17(8-9-21(19)30-23)29-18-10-11-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344532
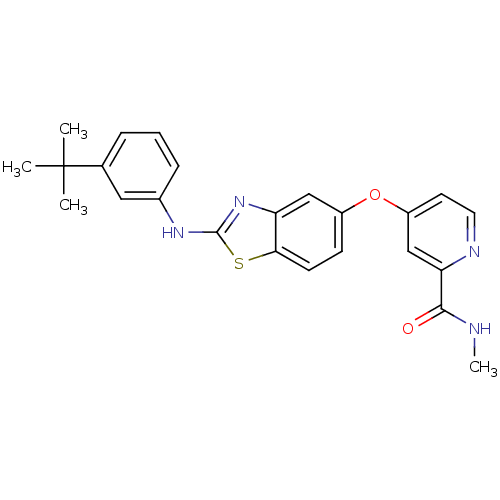
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O2S/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26034
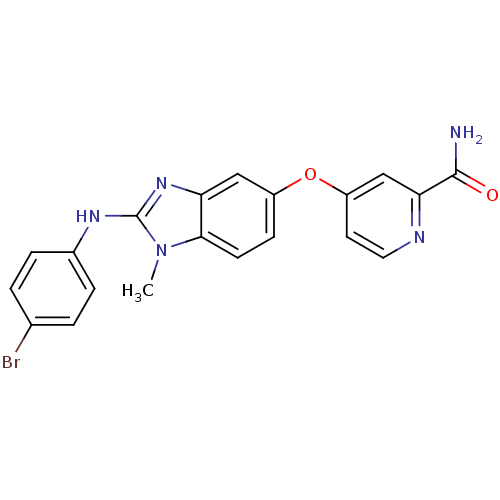
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(N)=O)ccc12 Show InChI InChI=1S/C20H16BrN5O2/c1-26-18-7-6-14(28-15-8-9-23-17(11-15)19(22)27)10-16(18)25-20(26)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500936
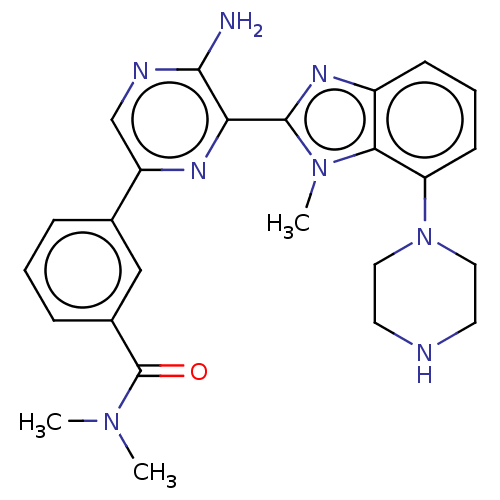
(CHEMBL3798762)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2n1C Show InChI InChI=1S/C25H28N8O/c1-31(2)25(34)17-7-4-6-16(14-17)19-15-28-23(26)21(29-19)24-30-18-8-5-9-20(22(18)32(24)3)33-12-10-27-11-13-33/h4-9,14-15,27H,10-13H2,1-3H3,(H2,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344525
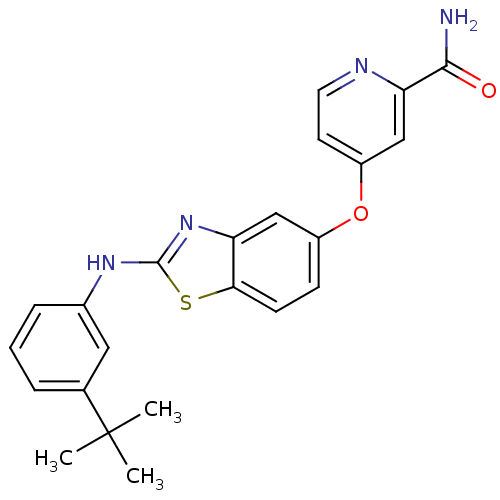
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CC(C)(C)c1cccc(Nc2nc3cc(Oc4ccnc(c4)C(N)=O)ccc3s2)c1 Show InChI InChI=1S/C23H22N4O2S/c1-23(2,3)14-5-4-6-15(11-14)26-22-27-18-12-16(7-8-20(18)30-22)29-17-9-10-25-19(13-17)21(24)28/h4-13H,1-3H3,(H2,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344548
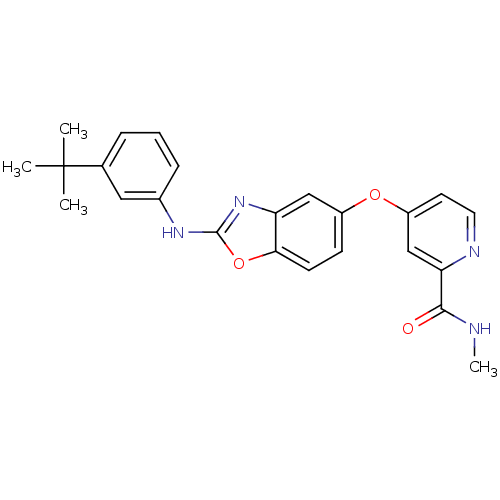
(4-(2-(3-tert-butyl phenylamino)benzo[d]oxazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O3/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26023
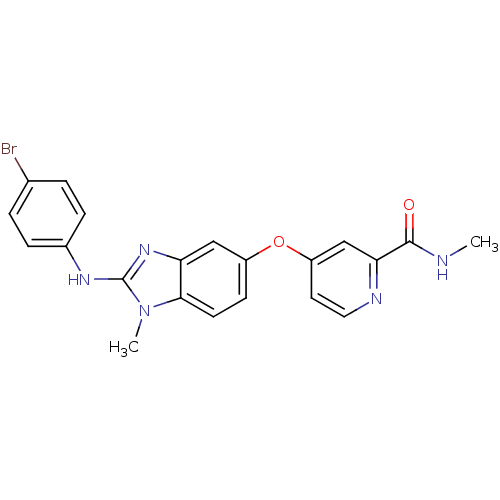
(4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(9-10-24-18)29-15-7-8-19-17(11-15)26-21(27(19)2)25-14-5-3-13(22)4-6-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500931

(CHEMBL3797873)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cccc(c3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C26H30N8O/c1-16-15-34(12-11-28-16)21-10-6-9-19-23(21)33(4)25(31-19)22-24(27)29-14-20(30-22)17-7-5-8-18(13-17)26(35)32(2)3/h5-10,13-14,16,28H,11-12,15H2,1-4H3,(H2,27,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500936

(CHEMBL3798762)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2n1C Show InChI InChI=1S/C25H28N8O/c1-31(2)25(34)17-7-4-6-16(14-17)19-15-28-23(26)21(29-19)24-30-18-8-5-9-20(22(18)32(24)3)33-12-10-27-11-13-33/h4-9,14-15,27H,10-13H2,1-3H3,(H2,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344539
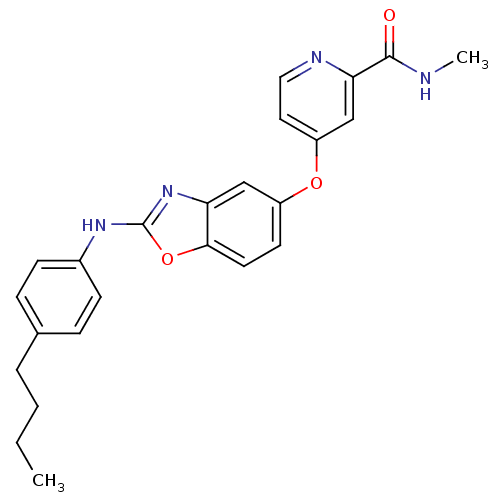
(4-(2-(4-butylphenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CCCCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3o2)cc1 Show InChI InChI=1S/C24H24N4O3/c1-3-4-5-16-6-8-17(9-7-16)27-24-28-20-14-18(10-11-22(20)31-24)30-19-12-13-26-21(15-19)23(29)25-2/h6-15H,3-5H2,1-2H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344538

(CHEMBL1778397 | N-methyl-4-(2-(4-(trifluoromethoxy...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H15F3N4O4/c1-25-19(29)17-11-15(8-9-26-17)30-14-6-7-18-16(10-14)28-20(31-18)27-12-2-4-13(5-3-12)32-21(22,23)24/h2-11H,1H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344530
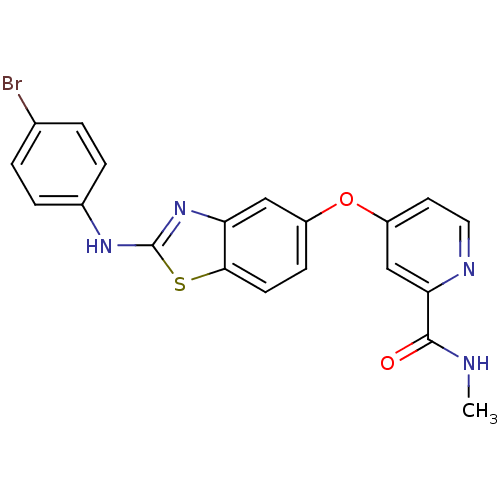
(4-(2-(4-bromophenylamino)benzo[d]thiazol-5-yloxy)-...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15BrN4O2S/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500933

(CHEMBL3797828)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(c1)-c1nc2cccc(N3CCNCC3)c2[nH]1 Show InChI InChI=1S/C25H27N7O/c1-31(2)25(33)17-6-3-5-16(13-17)18-14-19(23(26)28-15-18)24-29-20-7-4-8-21(22(20)30-24)32-11-9-27-10-12-32/h3-8,13-15,27H,9-12H2,1-2H3,(H2,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26023

(4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(9-10-24-18)29-15-7-8-19-17(11-15)26-21(27(19)2)25-14-5-3-13(22)4-6-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344543
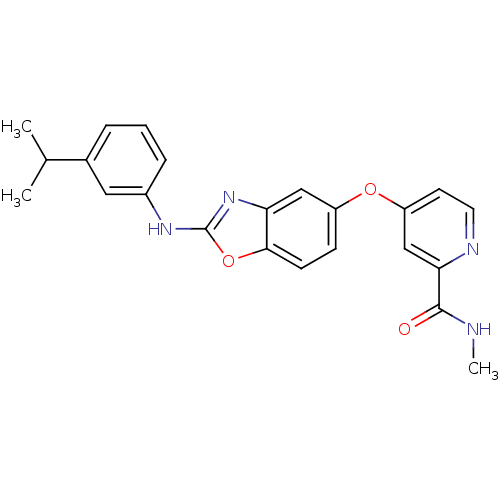
(4-(2-(3-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4cccc(c4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O3/c1-14(2)15-5-4-6-16(11-15)26-23-27-19-12-17(7-8-21(19)30-23)29-18-9-10-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344542

(4-(2-(4-bromophenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15BrN4O3/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344527

(4-(2-(4-isopropylphenylamino)benzo[d]thiazol-5-ylo...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(cc4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O2S/c1-14(2)15-4-6-16(7-5-15)26-23-27-19-12-17(8-9-21(19)30-23)29-18-10-11-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500934
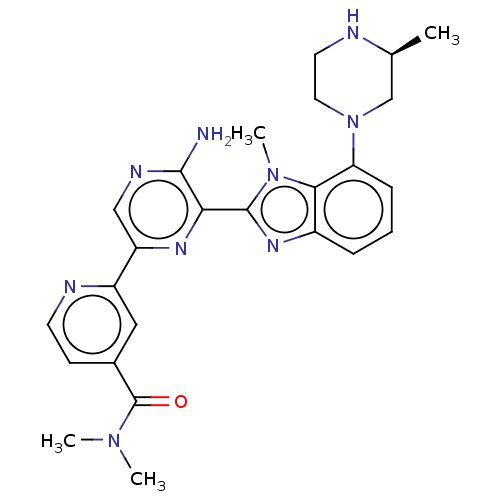
(CHEMBL3800585)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cc(ccn3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C25H29N9O/c1-15-14-34(11-10-27-15)20-7-5-6-17-22(20)33(4)24(31-17)21-23(26)29-13-19(30-21)18-12-16(8-9-28-18)25(35)32(2)3/h5-9,12-13,15,27H,10-11,14H2,1-4H3,(H2,26,29)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500934

(CHEMBL3800585)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cc(ccn3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C25H29N9O/c1-15-14-34(11-10-27-15)20-7-5-6-17-22(20)33(4)24(31-17)21-23(26)29-13-19(30-21)18-12-16(8-9-28-18)25(35)32(2)3/h5-9,12-13,15,27H,10-11,14H2,1-4H3,(H2,26,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26037
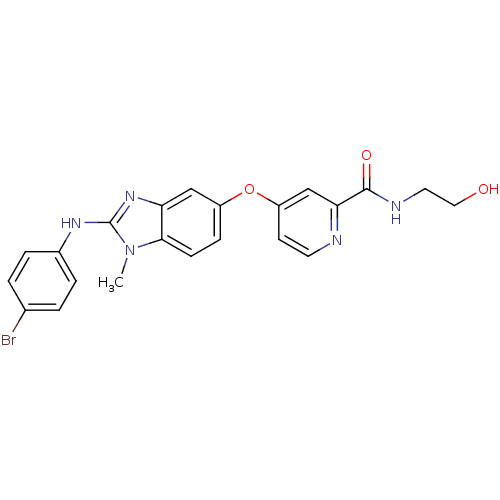
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCO)ccc12 Show InChI InChI=1S/C22H20BrN5O3/c1-28-20-7-6-16(31-17-8-9-24-19(13-17)21(30)25-10-11-29)12-18(20)27-22(28)26-15-4-2-14(23)3-5-15/h2-9,12-13,29H,10-11H2,1H3,(H,25,30)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26028
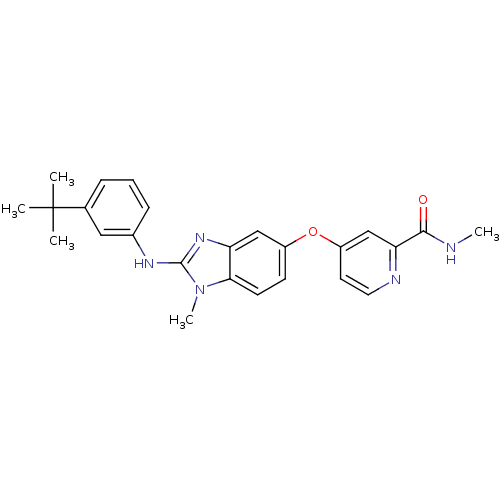
(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344526

(CHEMBL1778409 | N-methyl-4-(2-(4-(trifluoromethoxy...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H15F3N4O3S/c1-25-19(29)17-11-15(8-9-26-17)30-14-6-7-18-16(10-14)28-20(32-18)27-12-2-4-13(5-3-12)31-21(22,23)24/h2-11H,1H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500933

(CHEMBL3797828)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(c1)-c1nc2cccc(N3CCNCC3)c2[nH]1 Show InChI InChI=1S/C25H27N7O/c1-31(2)25(33)17-6-3-5-16(13-17)18-14-19(23(26)28-15-18)24-29-20-7-4-8-21(22(20)30-24)32-11-9-27-10-12-32/h3-8,13-15,27H,9-12H2,1-2H3,(H2,26,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26022
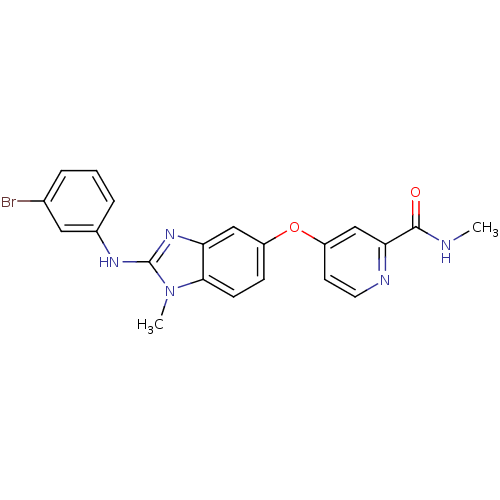
(4-({2-[(3-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(Br)c4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(8-9-24-18)29-15-6-7-19-17(11-15)26-21(27(19)2)25-14-5-3-4-13(22)10-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344531

(4-(2-(4-chlorophenylamino)benzo[d]thiazol-5-yloxy)...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(Cl)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15ClN4O2S/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344534

(4-(2-(3-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CC(C)c1cccc(Nc2nc3cc(Oc4ccnc(c4)C(N)=O)ccc3o2)c1 Show InChI InChI=1S/C22H20N4O3/c1-13(2)14-4-3-5-15(10-14)25-22-26-18-11-16(6-7-20(18)29-22)28-17-8-9-24-19(12-17)21(23)27/h3-13H,1-2H3,(H2,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26025

(N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(8-9-27-18)32-15-6-7-19-17(11-15)29-21(30(19)2)28-14-5-3-4-13(10-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26038

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCCC1CCCCN1NC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C28H31BrN6O2/c1-3-6-21-7-4-5-16-35(21)33-27(36)25-18-23(14-15-30-25)37-22-12-13-26-24(17-22)32-28(34(26)2)31-20-10-8-19(29)9-11-20/h8-15,17-18,21H,3-7,16H2,1-2H3,(H,31,32)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344529

(CHEMBL1778406 | N-methyl-4-(2-(p-tolylamino)benzo[...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(C)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18N4O2S/c1-13-3-5-14(6-4-13)24-21-25-17-11-15(7-8-19(17)28-21)27-16-9-10-23-18(12-16)20(26)22-2/h3-12H,1-2H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26025

(N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(8-9-27-18)32-15-6-7-19-17(11-15)29-21(30(19)2)28-14-5-3-4-13(10-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26017

(4-({2-[(3-tert-butylphenyl)amino]-1H-1,3-benzodiaz...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4cccc(c4)C(C)(C)C)[nH]c3c2)ccn1 Show InChI InChI=1S/C24H25N5O2/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-9-8-17(13-20(19)29-23)31-18-10-11-26-21(14-18)22(30)25-4/h5-14H,1-4H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26029
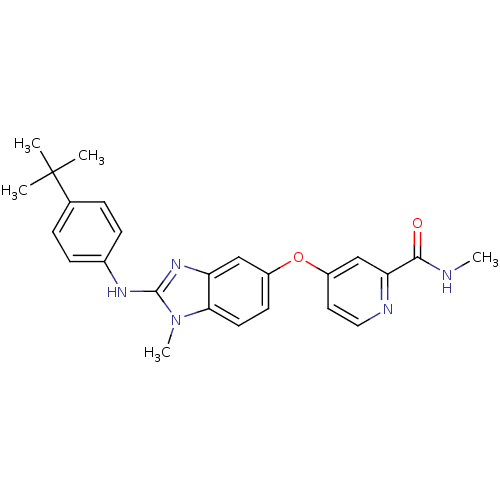
(4-({2-[(4-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(cc4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-6-8-17(9-7-16)28-24-29-20-14-18(10-11-22(20)30(24)5)32-19-12-13-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26026

(N-methyl-4-[(2-{methyl[4-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(cc4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(9-10-27-18)32-15-7-8-19-17(11-15)29-21(30(19)2)28-14-5-3-13(4-6-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344528

(4-(2-(4-butylphenylamino)benzo[d]thiazol-5-yloxy)-...)Show SMILES CCCCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3s2)cc1 Show InChI InChI=1S/C24H24N4O2S/c1-3-4-5-16-6-8-17(9-7-16)27-24-28-20-14-18(10-11-22(20)31-24)30-19-12-13-26-21(15-19)23(29)25-2/h6-15H,3-5H2,1-2H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26032
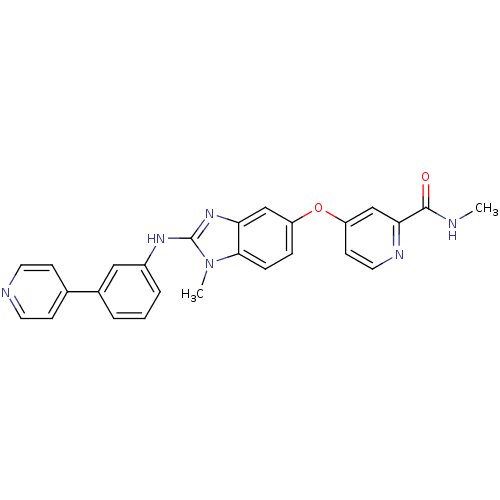
(N-methyl-4-[(2-{methyl[3-(pyridin-4-yl)phenyl]amin...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)-c4ccncc4)nc3c2)ccn1 Show InChI InChI=1S/C26H22N6O2/c1-27-25(33)23-16-21(10-13-29-23)34-20-6-7-24-22(15-20)31-26(32(24)2)30-19-5-3-4-18(14-19)17-8-11-28-12-9-17/h3-16H,1-2H3,(H,27,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26021
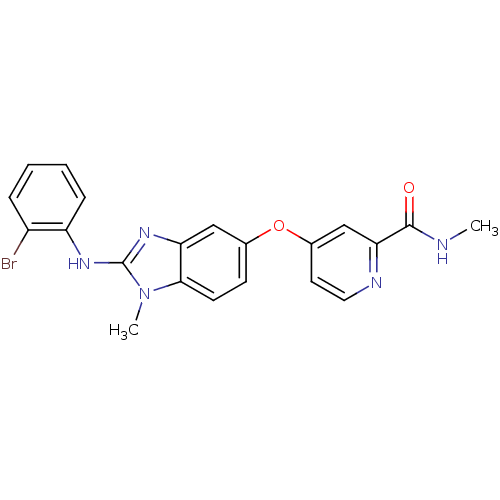
(4-({2-[(2-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccccc4Br)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-14(9-10-24-18)29-13-7-8-19-17(11-13)26-21(27(19)2)25-16-6-4-3-5-15(16)22/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26016

(N-methyl-4-[(2-{[4-(trifluoromethyl)phenyl]amino}-...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(F)(F)F)[nH]c3c2)ccn1 Show InChI InChI=1S/C21H16F3N5O2/c1-25-19(30)18-11-15(8-9-26-18)31-14-6-7-16-17(10-14)29-20(28-16)27-13-4-2-12(3-5-13)21(22,23)24/h2-11H,1H3,(H,25,30)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26037

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCO)ccc12 Show InChI InChI=1S/C22H20BrN5O3/c1-28-20-7-6-16(31-17-8-9-24-19(13-17)21(30)25-10-11-29)12-18(20)27-22(28)26-15-4-2-14(23)3-5-15/h2-9,12-13,29H,10-11H2,1H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344536

(4-(2-(4-(dimethylamino)phenylamino)benzo[d]oxazol-...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(cc4)N(C)C)nc3c2)ccn1 Show InChI InChI=1S/C22H21N5O3/c1-23-21(28)19-13-17(10-11-24-19)29-16-8-9-20-18(12-16)26-22(30-20)25-14-4-6-15(7-5-14)27(2)3/h4-13H,1-3H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26035
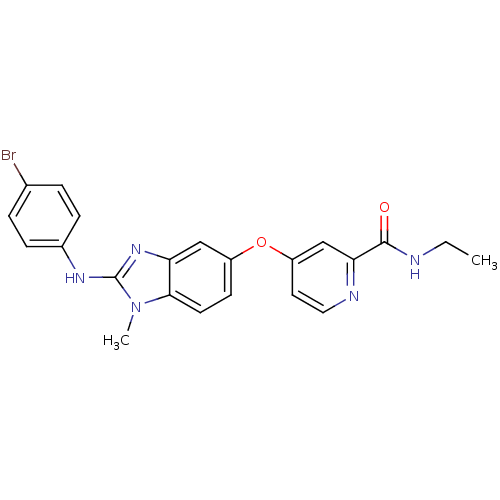
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H20BrN5O2/c1-3-24-21(29)19-13-17(10-11-25-19)30-16-8-9-20-18(12-16)27-22(28(20)2)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26032

(N-methyl-4-[(2-{methyl[3-(pyridin-4-yl)phenyl]amin...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)-c4ccncc4)nc3c2)ccn1 Show InChI InChI=1S/C26H22N6O2/c1-27-25(33)23-16-21(10-13-29-23)34-20-6-7-24-22(15-20)31-26(32(24)2)30-19-5-3-4-18(14-19)17-8-11-28-12-9-17/h3-16H,1-2H3,(H,27,33)(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26035

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H20BrN5O2/c1-3-24-21(29)19-13-17(10-11-25-19)30-16-8-9-20-18(12-16)27-22(28(20)2)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26020
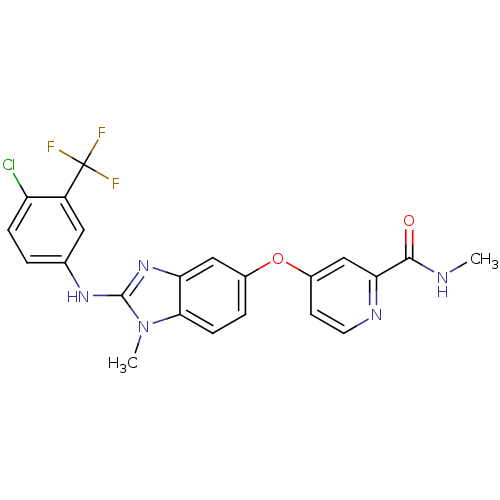
(4-[(2-{[4-chloro-3-(trifluoromethyl)phenyl](methyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Cl)c(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H17ClF3N5O2/c1-27-20(32)18-11-14(7-8-28-18)33-13-4-6-19-17(10-13)30-21(31(19)2)29-12-3-5-16(23)15(9-12)22(24,25)26/h3-11H,1-2H3,(H,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26018

(4-({2-[(4-tert-butylphenyl)amino]-1H-1,3-benzodiaz...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(C)(C)C)[nH]c3c2)ccn1 Show InChI InChI=1S/C24H25N5O2/c1-24(2,3)15-5-7-16(8-6-15)27-23-28-19-10-9-17(13-20(19)29-23)31-18-11-12-26-21(14-18)22(30)25-4/h5-14H,1-4H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26036
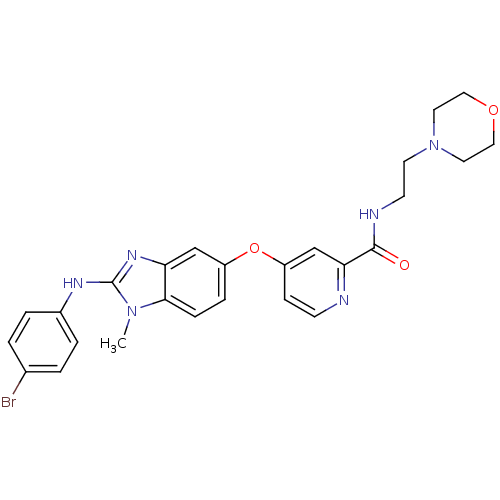
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCN3CCOCC3)ccc12 Show InChI InChI=1S/C26H27BrN6O3/c1-32-24-7-6-20(16-22(24)31-26(32)30-19-4-2-18(27)3-5-19)36-21-8-9-28-23(17-21)25(34)29-10-11-33-12-14-35-15-13-33/h2-9,16-17H,10-15H2,1H3,(H,29,34)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
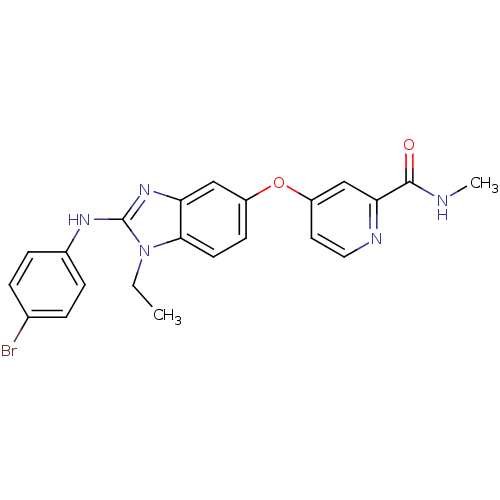
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26033
(4-({2-[(4-bromophenyl)(ethyl)amino]-1H-1,3-benzodi...)Show SMILES CCn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NC)ccc12 Show InChI InChI=1S/C22H20BrN5O2/c1-3-28-20-9-8-16(30-17-10-11-25-19(13-17)21(29)24-2)12-18(20)27-22(28)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data