 Found 62 hits with Last Name = 'aivazian' and Initial = 'd'
Found 62 hits with Last Name = 'aivazian' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50352206
(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using a fluorescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352202

(CHEMBL1825101)Show SMILES CS(=O)(=O)N(CCN)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C20H28N8O2S/c1-31(29,30)27(11-10-21)18-9-8-16(14-22-18)25-20-23-12-15-13-24-28(19(15)26-20)17-6-4-2-3-5-7-17/h8-9,12-14,17H,2-7,10-11,21H2,1H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352200

(CHEMBL1825100)Show SMILES CS(=O)(=O)N(CCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C20H27N7O3S/c1-31(29,30)26(10-11-28)18-9-8-16(14-21-18)24-20-22-12-15-13-23-27(19(15)25-20)17-6-4-2-3-5-7-17/h8-9,12-14,17,28H,2-7,10-11H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352215

(CHEMBL1825093)Show SMILES CS(=O)(=O)N(CCN)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C21H29N7O2S/c1-31(29,30)27(13-12-22)18-10-8-17(9-11-18)25-21-23-14-16-15-24-28(20(16)26-21)19-6-4-2-3-5-7-19/h8-11,14-15,19H,2-7,12-13,22H2,1H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352208
(CHEMBL1825090)Show SMILES CS(=O)(=O)N(CCCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C26H37N7O3S/c1-37(34,35)32(14-6-13-31-15-17-36-18-16-31)23-11-9-22(10-12-23)29-26-27-19-21-20-28-33(25(21)30-26)24-7-4-2-3-5-8-24/h9-12,19-20,24H,2-8,13-18H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352209

(CHEMBL1825092)Show SMILES CC(C)N(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1)S(C)(=O)=O Show InChI InChI=1S/C22H30N6O2S/c1-16(2)28(31(3,29)30)20-12-10-18(11-13-20)25-22-23-14-17-15-24-27(21(17)26-22)19-8-6-4-5-7-9-19/h10-16,19H,4-9H2,1-3H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352208

(CHEMBL1825090)Show SMILES CS(=O)(=O)N(CCCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C26H37N7O3S/c1-37(34,35)32(14-6-13-31-15-17-36-18-16-31)23-11-9-22(10-12-23)29-26-27-19-21-20-28-33(25(21)30-26)24-7-4-2-3-5-8-24/h9-12,19-20,24H,2-8,13-18H2,1H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352212

(CHEMBL1821761)Show SMILES CS(=O)(=O)N(CCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C21H28N6O3S/c1-31(29,30)26(12-13-28)18-10-8-17(9-11-18)24-21-22-14-16-15-23-27(20(16)25-21)19-6-4-2-3-5-7-19/h8-11,14-15,19,28H,2-7,12-13H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352210

(CHEMBL1825091)Show SMILES CS(=O)(=O)N(CCCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C22H30N6O3S/c1-32(30,31)27(13-6-14-29)19-11-9-18(10-12-19)25-22-23-15-17-16-24-28(21(17)26-22)20-7-4-2-3-5-8-20/h9-12,15-16,20,29H,2-8,13-14H2,1H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352210

(CHEMBL1825091)Show SMILES CS(=O)(=O)N(CCCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C22H30N6O3S/c1-32(30,31)27(13-6-14-29)19-11-9-18(10-12-19)25-22-23-15-17-16-24-28(21(17)26-22)20-7-4-2-3-5-8-20/h9-12,15-16,20,29H,2-8,13-14H2,1H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352211
(CHEMBL1825095)Show SMILES CS(=O)(=O)N(CCCN1CCCCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C27H39N7O2S/c1-37(35,36)33(19-9-18-32-16-7-4-8-17-32)24-14-12-23(13-15-24)30-27-28-20-22-21-29-34(26(22)31-27)25-10-5-2-3-6-11-25/h12-15,20-21,25H,2-11,16-19H2,1H3,(H,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352205
(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352211

(CHEMBL1825095)Show SMILES CS(=O)(=O)N(CCCN1CCCCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C27H39N7O2S/c1-37(35,36)33(19-9-18-32-16-7-4-8-17-32)24-14-12-23(13-15-24)30-27-28-20-22-21-29-34(26(22)31-27)25-10-5-2-3-6-11-25/h12-15,20-21,25H,2-11,16-19H2,1H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352203

(CHEMBL1825099)Show SMILES CN(C)CCN(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1)S(C)(=O)=O Show InChI InChI=1S/C22H32N8O2S/c1-28(2)12-13-29(33(3,31)32)20-11-10-18(16-23-20)26-22-24-14-17-15-25-30(21(17)27-22)19-8-6-4-5-7-9-19/h10-11,14-16,19H,4-9,12-13H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352204
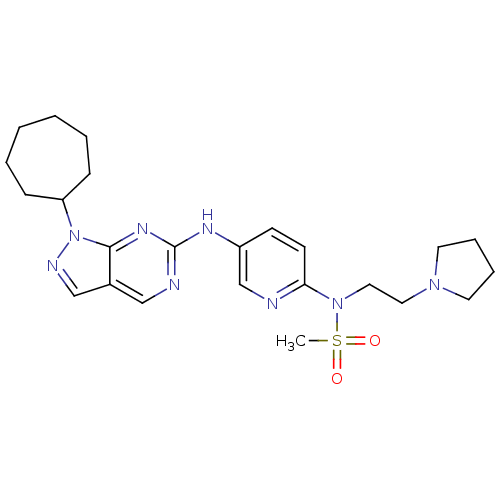
(CHEMBL1825098)Show SMILES CS(=O)(=O)N(CCN1CCCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H34N8O2S/c1-35(33,34)31(15-14-30-12-6-7-13-30)22-11-10-20(18-25-22)28-24-26-16-19-17-27-32(23(19)29-24)21-8-4-2-3-5-9-21/h10-11,16-18,21H,2-9,12-15H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352214
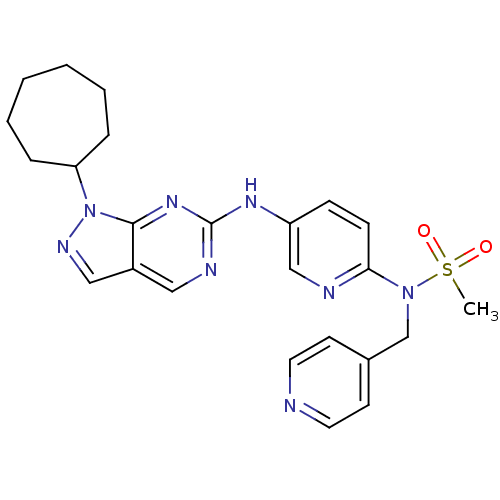
(CHEMBL1825102)Show SMILES CS(=O)(=O)N(Cc1ccncc1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H28N8O2S/c1-35(33,34)31(17-18-10-12-25-13-11-18)22-9-8-20(16-26-22)29-24-27-14-19-15-28-32(23(19)30-24)21-6-4-2-3-5-7-21/h8-16,21H,2-7,17H2,1H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352212

(CHEMBL1821761)Show SMILES CS(=O)(=O)N(CCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C21H28N6O3S/c1-31(29,30)26(12-13-28)18-10-8-17(9-11-18)24-21-22-14-16-15-23-27(20(16)25-21)19-6-4-2-3-5-7-19/h8-11,14-15,19,28H,2-7,12-13H2,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352215

(CHEMBL1825093)Show SMILES CS(=O)(=O)N(CCN)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C21H29N7O2S/c1-31(29,30)27(13-12-22)18-10-8-17(9-11-18)25-21-23-14-16-15-24-28(20(16)26-21)19-6-4-2-3-5-7-19/h8-11,14-15,19H,2-7,12-13,22H2,1H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352213
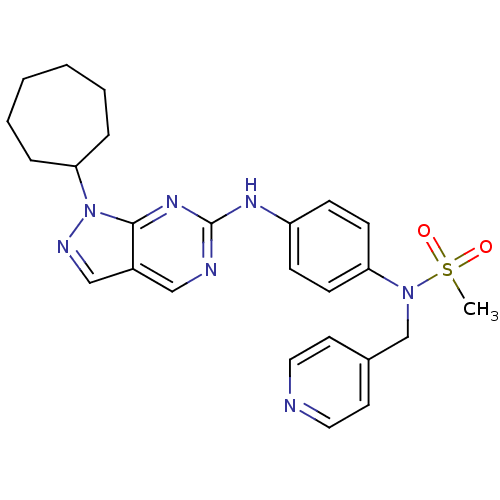
(CHEMBL1825094)Show SMILES CS(=O)(=O)N(Cc1ccncc1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H29N7O2S/c1-35(33,34)31(18-19-12-14-26-15-13-19)22-10-8-21(9-11-22)29-25-27-16-20-17-28-32(24(20)30-25)23-6-4-2-3-5-7-23/h8-17,23H,2-7,18H2,1H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352213

(CHEMBL1825094)Show SMILES CS(=O)(=O)N(Cc1ccncc1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H29N7O2S/c1-35(33,34)31(18-19-12-14-26-15-13-19)22-10-8-21(9-11-22)29-25-27-16-20-17-28-32(24(20)30-25)23-6-4-2-3-5-7-23/h8-17,23H,2-7,18H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352201

(CHEMBL1825097)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H34N8O3S/c1-36(33,34)31(11-10-30-12-14-35-15-13-30)22-9-8-20(18-25-22)28-24-26-16-19-17-27-32(23(19)29-24)21-6-4-2-3-5-7-21/h8-9,16-18,21H,2-7,10-15H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using a fluorescent probe 7-benzyloxyquinoline |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using a fluorescent probe 7-benzyloxy-4(trifluoromethyl)-coumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM17051

(BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...)Show SMILES Ic1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)c1cccs1 Show InChI InChI=1S/C23H26IN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cGAS in human THP1 cells assessed as reduction in salmon sperm dsDNA-induced IFN-beta expression preincubated for 1 hr followed by dsDN... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352209

(CHEMBL1825092)Show SMILES CC(C)N(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1)S(C)(=O)=O Show InChI InChI=1S/C22H30N6O2S/c1-16(2)28(31(3,29)30)20-12-10-18(11-13-20)25-22-23-14-17-15-24-27(21(17)26-22)19-8-6-4-5-7-9-19/h10-16,19H,4-9H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352214

(CHEMBL1825102)Show SMILES CS(=O)(=O)N(Cc1ccncc1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H28N8O2S/c1-35(33,34)31(17-18-10-12-25-13-11-18)22-9-8-20(16-26-22)29-24-27-14-19-15-28-32(23(19)30-24)21-6-4-2-3-5-7-21/h8-16,21H,2-7,17H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352200

(CHEMBL1825100)Show SMILES CS(=O)(=O)N(CCO)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C20H27N7O3S/c1-31(29,30)26(10-11-28)18-9-8-16(14-21-18)24-20-22-12-15-13-23-27(19(15)25-20)17-6-4-2-3-5-7-17/h8-9,12-14,17,28H,2-7,10-11H2,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352201

(CHEMBL1825097)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H34N8O3S/c1-36(33,34)31(11-10-30-12-14-35-15-13-30)22-9-8-20(18-25-22)28-24-26-16-19-17-27-32(23(19)29-24)21-6-4-2-3-5-7-21/h8-9,16-18,21H,2-7,10-15H2,1H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352202

(CHEMBL1825101)Show SMILES CS(=O)(=O)N(CCN)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C20H28N8O2S/c1-31(29,30)27(11-10-21)18-9-8-16(14-22-18)25-20-23-12-15-13-24-28(19(15)26-20)17-6-4-2-3-5-7-17/h8-9,12-14,17H,2-7,10-11,21H2,1H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352203

(CHEMBL1825099)Show SMILES CN(C)CCN(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1)S(C)(=O)=O Show InChI InChI=1S/C22H32N8O2S/c1-28(2)12-13-29(33(3,31)32)20-11-10-18(16-23-20)26-22-24-14-17-15-25-30(21(17)27-22)19-8-6-4-5-7-9-19/h10-11,14-16,19H,4-9,12-13H2,1-3H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352204

(CHEMBL1825098)Show SMILES CS(=O)(=O)N(CCN1CCCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C24H34N8O2S/c1-35(33,34)31(15-14-30-12-6-7-13-30)22-11-10-20(18-25-22)28-24-26-16-19-17-27-32(23(19)29-24)21-8-4-2-3-5-9-21/h10-11,16-18,21H,2-9,12-15H2,1H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250108
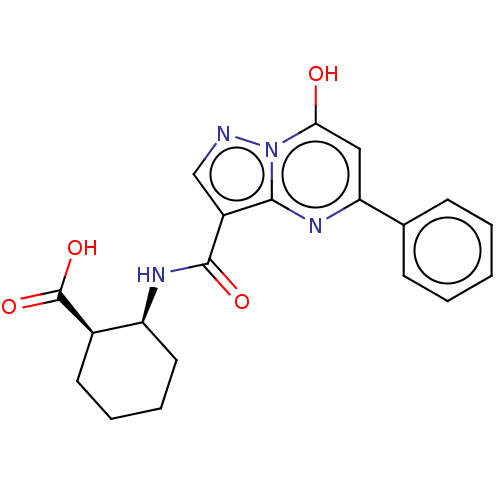
(CHEMBL4096573)Show SMILES OC(=O)[C@@H]1CCCC[C@@H]1NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O4/c25-17-10-16(12-6-2-1-3-7-12)22-18-14(11-21-24(17)18)19(26)23-15-9-5-4-8-13(15)20(27)28/h1-3,6-7,10-11,13,15,25H,4-5,8-9H2,(H,23,26)(H,27,28)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250108

(CHEMBL4096573)Show SMILES OC(=O)[C@@H]1CCCC[C@@H]1NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O4/c25-17-10-16(12-6-2-1-3-7-12)22-18-14(11-21-24(17)18)19(26)23-15-9-5-4-8-13(15)20(27)28/h1-3,6-7,10-11,13,15,25H,4-5,8-9H2,(H,23,26)(H,27,28)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250111

(CHEMBL4084664)Show SMILES C[C@@H](CO)NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C16H16N4O3/c1-10(9-21)18-16(23)12-8-17-20-14(22)7-13(19-15(12)20)11-5-3-2-4-6-11/h2-8,10,21-22H,9H2,1H3,(H,18,23)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using a fluorescent probe 7-benzyloxy-4(trifluoromethyl)-coumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using a fluorescent probe 7-benzyloxyquinoline |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50352207

(CHEMBL1825103)Show SMILES CS(=O)(=O)N(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)nc1)S(C)(=O)=O Show InChI InChI=1S/C19H25N7O4S2/c1-31(27,28)26(32(2,29)30)16-9-10-17(20-13-16)23-19-21-11-14-12-22-25(18(14)24-19)15-7-5-3-4-6-8-15/h9-13,15H,3-8H2,1-2H3,(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B assessed as phosphorylation of Z-lyte Peptide at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using a fluorescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 using a fluorescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4methylcoumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 using a fluorescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4methylcoumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50352207

(CHEMBL1825103)Show SMILES CS(=O)(=O)N(c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)nc1)S(C)(=O)=O Show InChI InChI=1S/C19H25N7O4S2/c1-31(27,28)26(32(2,29)30)16-9-10-17(20-13-16)23-19-21-11-14-12-22-25(18(14)24-19)15-7-5-3-4-6-8-15/h9-13,15H,3-8H2,1-2H3,(H,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A assessed as phosphorylation of Lats2 substrate at 0.017 to 30 nM by FRET assay |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50352205

(CHEMBL1825096)Show SMILES CS(=O)(=O)Nc1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cn1 Show InChI InChI=1S/C18H23N7O2S/c1-28(26,27)24-16-9-8-14(12-19-16)22-18-20-10-13-11-21-25(17(13)23-18)15-6-4-2-3-5-7-15/h8-12,15H,2-7H2,1H3,(H,19,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50352206

(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250109

(CHEMBL4062994)Show InChI InChI=1S/C15H12N4O4/c20-12-6-11(9-4-2-1-3-5-9)18-14-10(7-17-19(12)14)15(23)16-8-13(21)22/h1-7,20H,8H2,(H,16,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250106
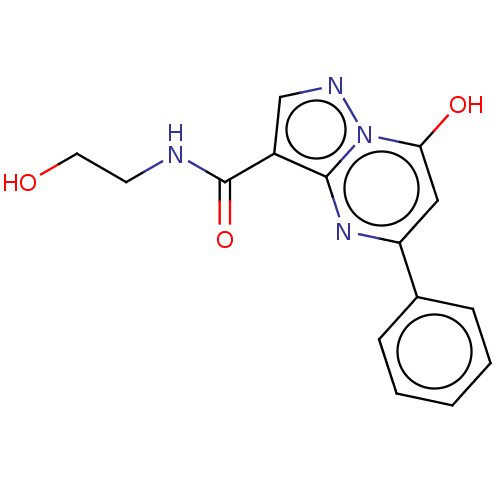
(CHEMBL4103560)Show InChI InChI=1S/C15H14N4O3/c20-7-6-16-15(22)11-9-17-19-13(21)8-12(18-14(11)19)10-4-2-1-3-5-10/h1-5,8-9,20-21H,6-7H2,(H,16,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data