

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2685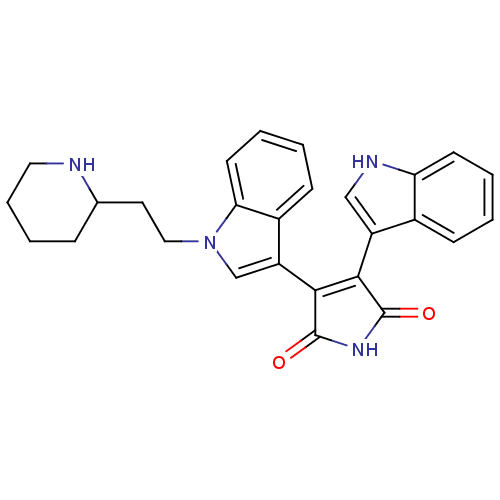 (3-(1H-indol-3-yl)-4-{1-[2-(piperidin-2-yl)ethyl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2683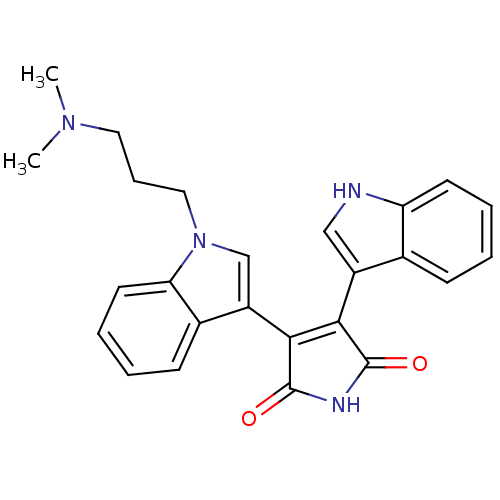 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2694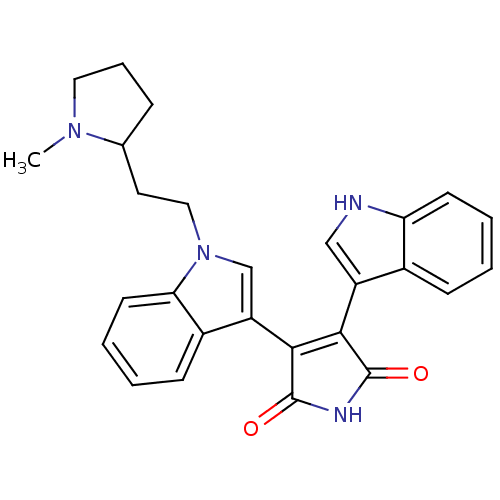 (3-(1H-indol-3-yl)-4-{1-[2-(1-methylpyrrolidin-2-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2691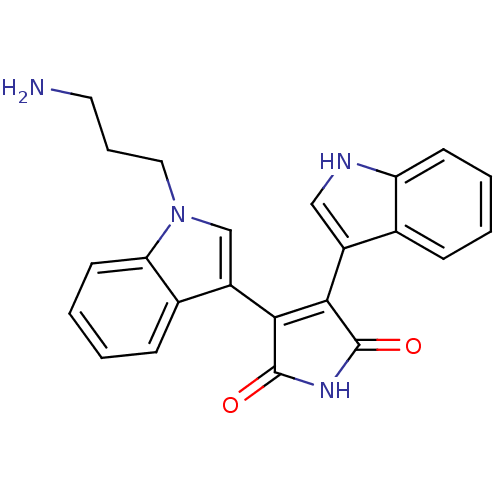 (3-[1-(3-aminopropyl)-1H-indol-3-yl]-4-(1H-indol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2681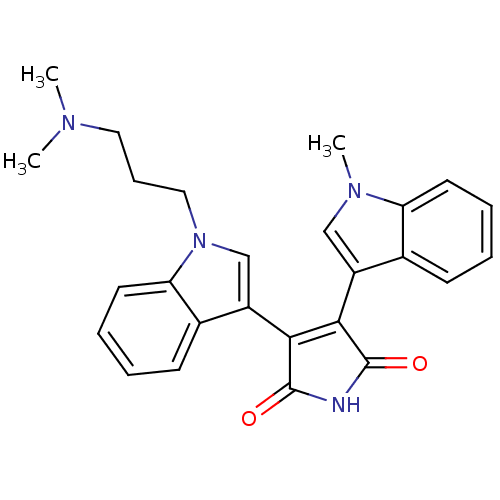 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2692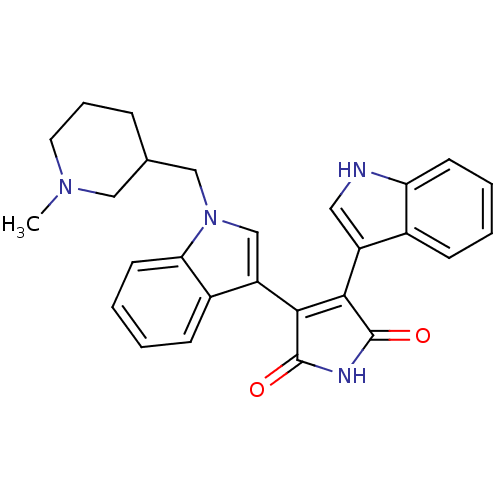 (3-(1H-indol-3-yl)-4-{1-[(1-methylpiperidin-3-yl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173927 (CHEMBL3808902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173927 (CHEMBL3808902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2686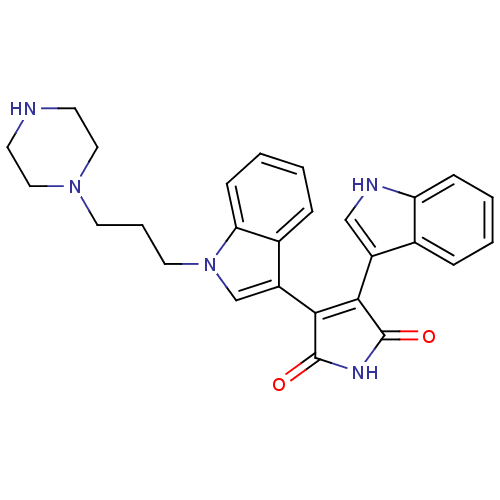 (3-(1H-indol-3-yl)-4-{1-[3-(piperazin-1-yl)propyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173926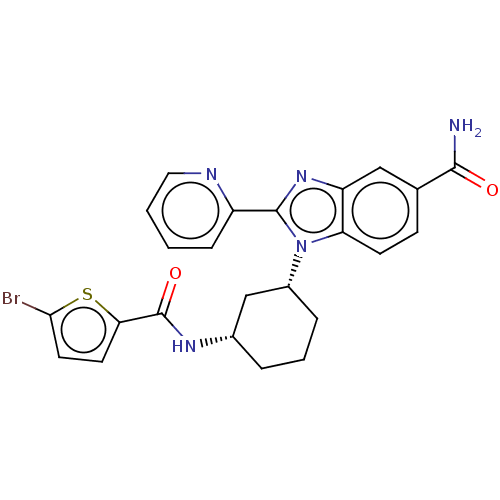 (CHEMBL3808971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173928 (CHEMBL3809839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173926 (CHEMBL3808971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173928 (CHEMBL3809839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2693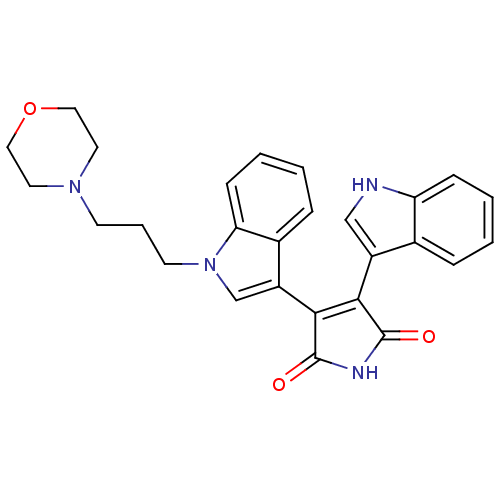 (3-(1H-indol-3-yl)-4-{1-[3-(morpholin-4-yl)propyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173923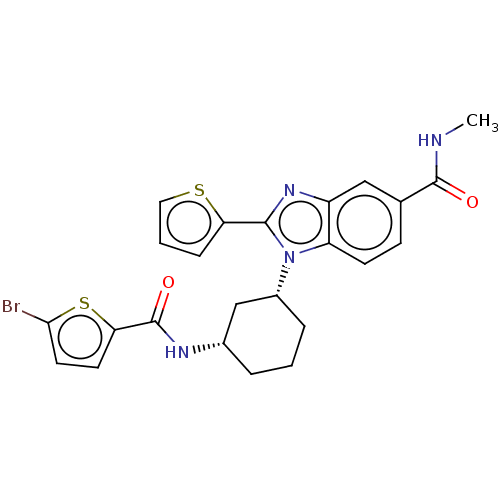 (CHEMBL3810317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2690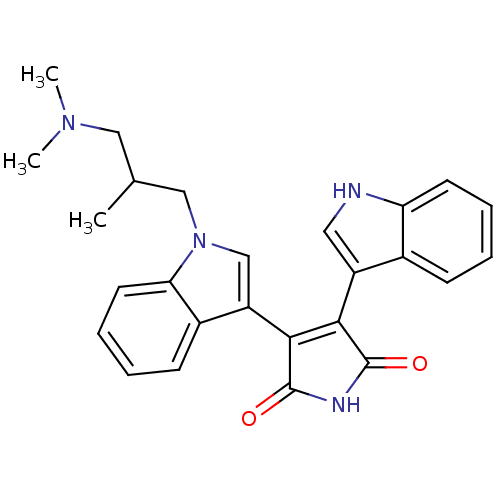 (3-(1-{2-[(dimethylamino)methyl]propyl}-1H-indol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173923 (CHEMBL3810317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173922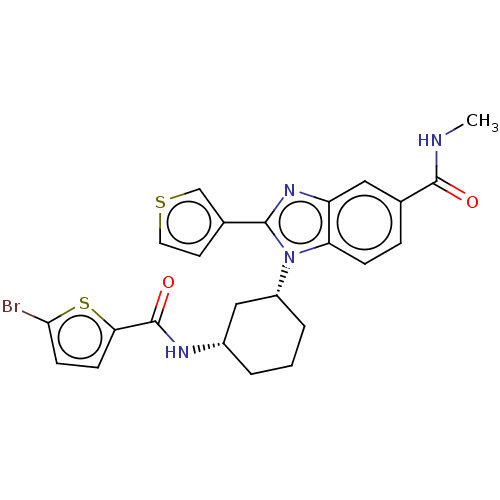 (CHEMBL3809282) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2682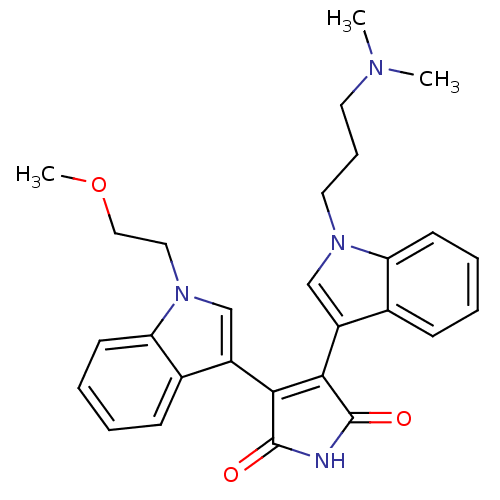 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173922 (CHEMBL3809282) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2583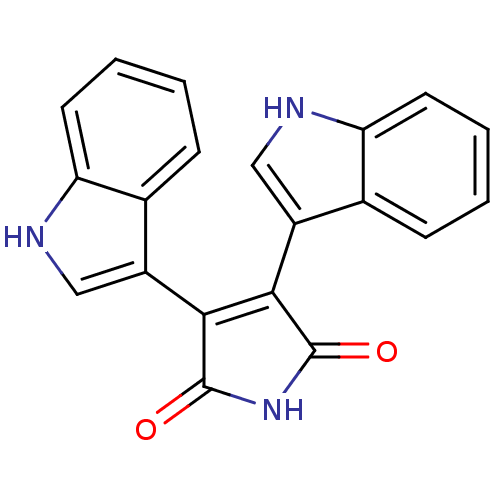 (3,4-Bis(3-indolyl)-1H-pyrrole-2,5-dione | 3,4-bis(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 15 (Homo sapiens (Human)) | BDBM50548060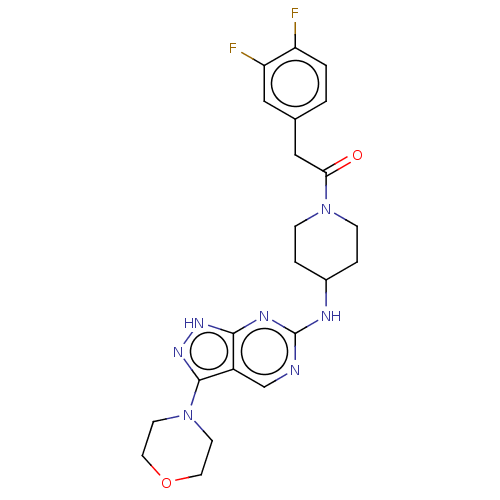 (CHEMBL4762609) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERK8 (2 to 544 residues) incubated for 5 mins in presence of [gamma33P]ATP by scintillation counting analysis | Citation and Details Article DOI: 10.1039/d0md00203h BindingDB Entry DOI: 10.7270/Q2QJ7MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50384600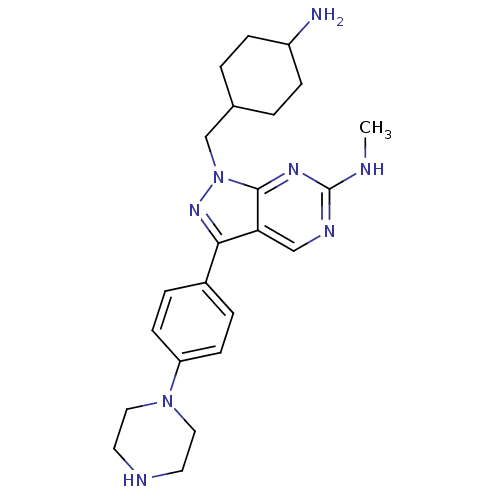 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK2 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173939 (CHEMBL3809237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform (Oryctolagus cuniculus (rabbit)) | BDBM2691 (3-[1-(3-aminopropyl)-1H-indol-3-yl]-4-(1H-indol-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PK is measured by its ability to transfer from [gamma-32P]ATP to phosphorylase b. | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50384600 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK4 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2680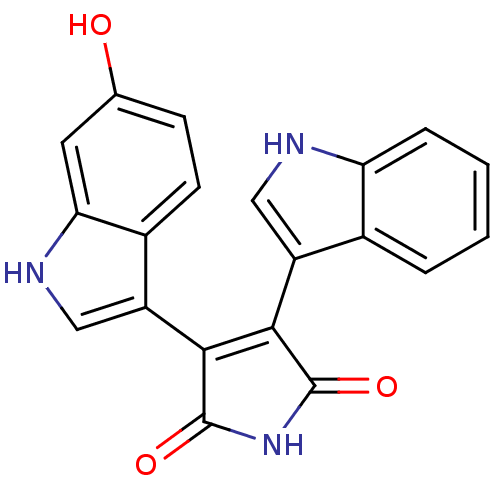 (3-(6-hydroxy-1H-indol-3-yl)-4-(1H-indol-3-yl)-2,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform (Oryctolagus cuniculus (rabbit)) | BDBM2684 (3-[3-(dimethylamino)propyl]-3,13,23-triazahexacycl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PK is measured by its ability to transfer from [gamma-32P]ATP to phosphorylase b. | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2687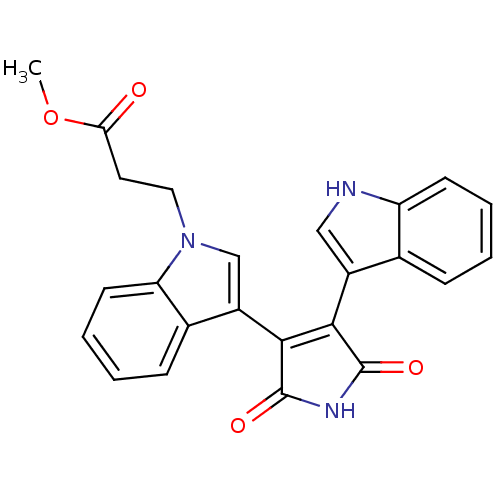 (Bisindolyl deriv. 13 | methyl 3-{3-[4-(1H-indol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2688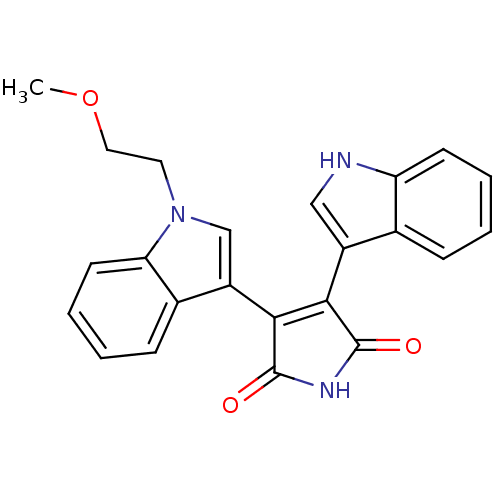 (3-(1H-indol-3-yl)-4-[1-(2-methoxyethyl)-1H-indol-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173933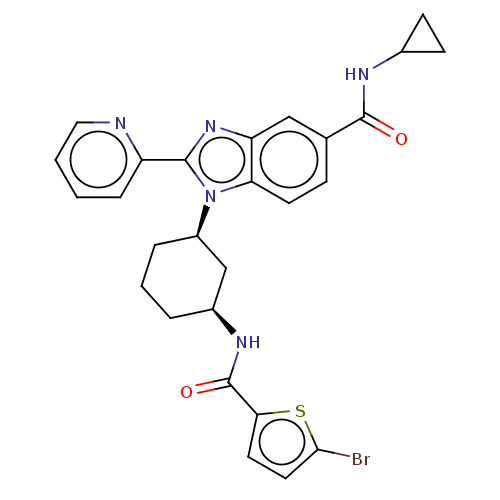 (CHEMBL3808894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441159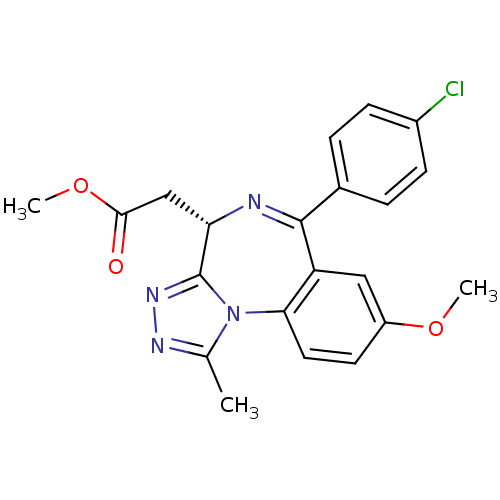 (CHEMBL2430882) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173918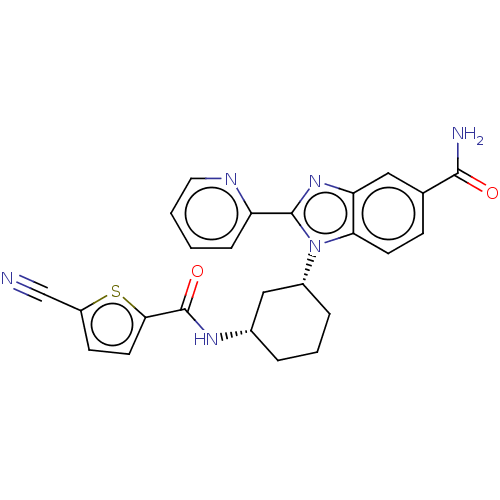 (CHEMBL3809916) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173916 (CHEMBL3809321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173939 (CHEMBL3809237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2684 (3-[3-(dimethylamino)propyl]-3,13,23-triazahexacycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKC, activated by phosphatidylserine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich hi... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441168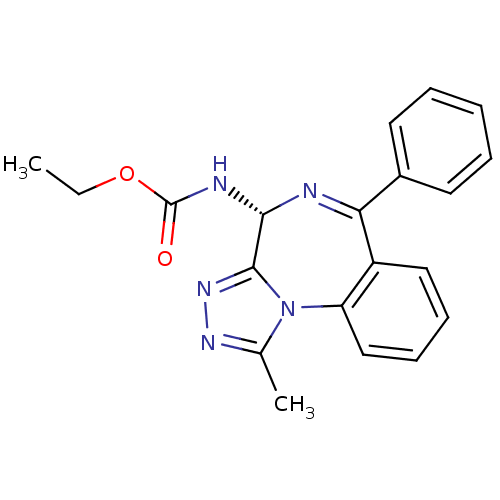 (CHEMBL2430873) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441164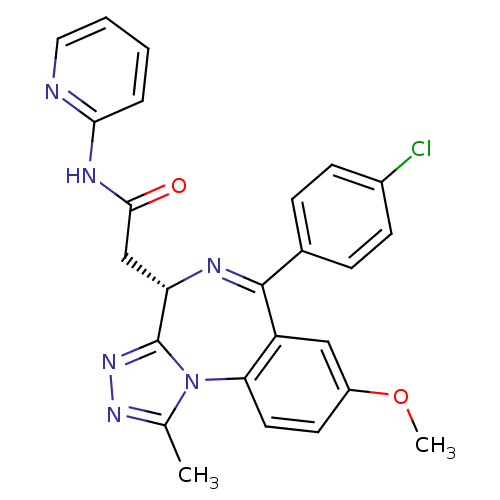 (CHEMBL2430877) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441165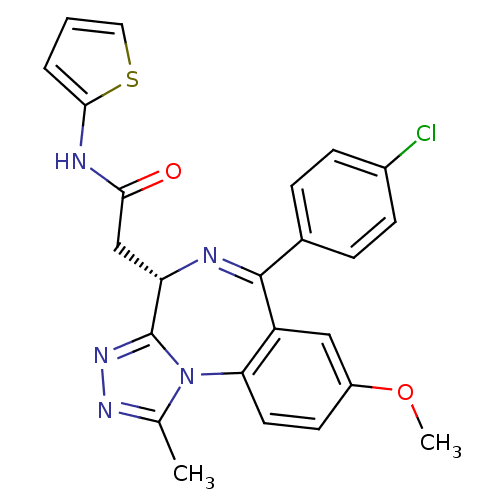 (CHEMBL2430876) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441158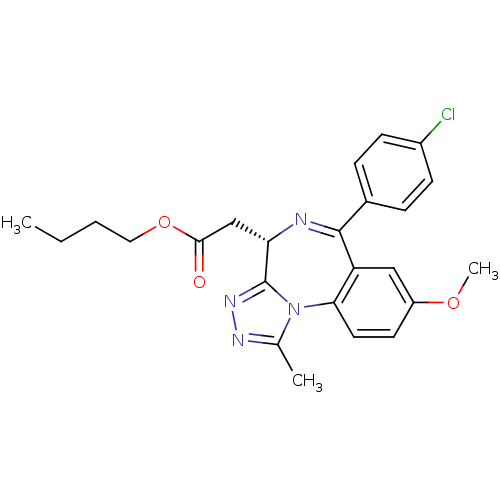 (CHEMBL2430883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform (Oryctolagus cuniculus (rabbit)) | BDBM2685 (3-(1H-indol-3-yl)-4-{1-[2-(piperidin-2-yl)ethyl]-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PK is measured by its ability to transfer from [gamma-32P]ATP to phosphorylase b. | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Rattus norvegicus (rat)) | BDBM2691 (3-[1-(3-aminopropyl)-1H-indol-3-yl]-4-(1H-indol-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Rattus norvegicus (rat)) | BDBM2684 (3-[3-(dimethylamino)propyl]-3,13,23-triazahexacycl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Glaxo | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441166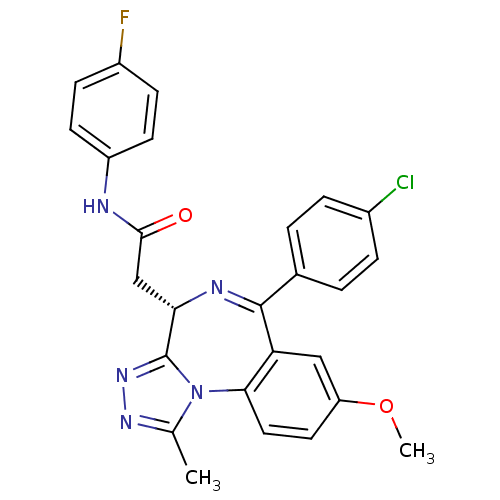 (CHEMBL2430875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441171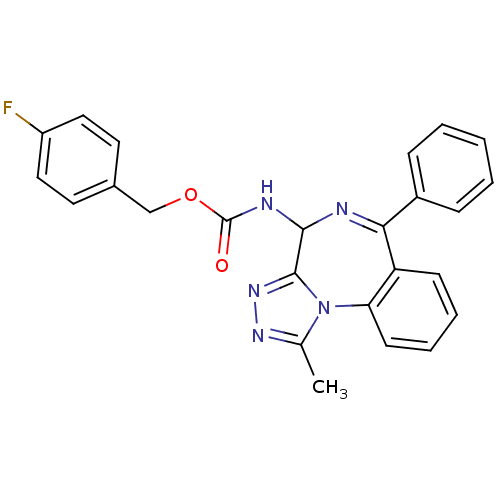 (CHEMBL2430869) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441176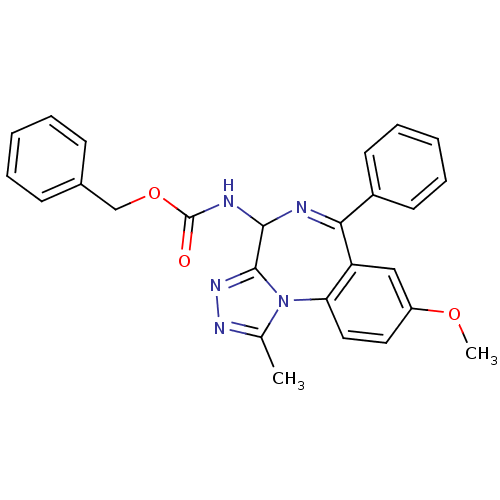 (CHEMBL2430892) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365264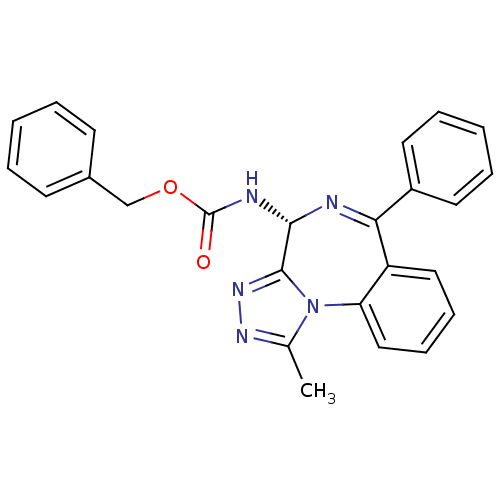 (CHEMBL1738926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50441168 (CHEMBL2430873) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD3 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173925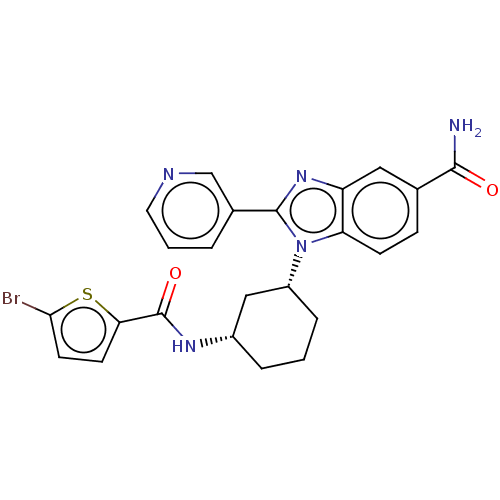 (CHEMBL3808495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50441162 (CHEMBL2430879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Binding affinity to His6-tagged BRD4 (unknown origin) after 60 mins by fluorescence anisotropy binding Assay | J Med Chem 56: 7501-15 (2013) Article DOI: 10.1021/jm401088k BindingDB Entry DOI: 10.7270/Q2SX6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 169 total ) | Next | Last >> |